Abstract
Purpose
Although cisplatin is the drug of choice in treating lung cancer patients, relapse and resistance is a common drawback to its clinical effectiveness. Based on cisplatin’s reported ability to interfere with numerous cellular components, including mitochondria, we probed alterations in metabolism in cisplatin-resistant tumor cell lines to reveal targets for overcoming this important form of resistance.
Methods
Cisplatin-resistant lung and ovarian cancer cell lines were used to evaluate the efficacy of metabolic inhibitors for selectively targeting cisplatin-resistant cells under varying oxygen conditions.
Results
Three cisplatin-resistant cancer cell lines expressed lower HKII protein when compared to the respective cisplatin-sensitive cancer cell lines from which they were derived. Under anaerobic and hypoxic conditions, treatment with the glycolytic inhibitors 2-deoxyglucose (2-DG) and 2-fluorodeoxyglucose (2-FDG) correlated with increased cytotoxicity and more pronounced decreases in lactate production in cisplatin-resistant cells, indicating a greater blockage of glycolysis. Knockdown of HKI or HKII with siRNA in the parental lung cancer cell lines led to increased 2-FDG-induced cell death under anaerobic conditions. Under normal oxygen conditions, blockage of either fatty acid oxidation or deprivation of glutamine resulted in cell death in cisplatin-resistant lung cancer cell lines.
Conclusions
Altered hexokinase levels in cisplatin-resistant cancer cell lines leads to increased sensitivity to glycolytic inhibition under anaerobic conditions, whereas under normoxic conditions, blockage of either fatty acid oxidation or deprivation of glutamine leads to cell death. These findings may be clinically applicable when considering cisplatin resistance.







Similar content being viewed by others
References
Yang Z, Schumaker L, Egorin M, Zuhowski E, Guo Z, Cullen KJ (2006) Cisplatin preferentially binds mitochondrial DNA and voltage-dependent anion channel protein in the mitochondrial membrane of head and neck squamous cell carcinoma: possible role in apoptosis. Clin Cancer Res 12(19):5817–5825. doi:10.1158/1078-0432
Leyton J, Latigo JR, Perumal M, Dhaliwal H, He Q, Aboagye EO (2005) Early detection of tumor response to chemotherapy by 3′ -Deoxy-3′-[18F]fluorothymidine positron emission tomography: the effect of cisplatin on a fibrosarcoma tumor model In vivo. Cancer Res 65:4202–4210. doi:10.1158/0008-5472.CAN-04-4008
Warburg O, Wind F, Negelein E (1927) Metabolism of Tumors in the Body. J Gen Physiol 8(6):519–530
Hanahan D, Weinberg R (2011) Hallmarks of cancer: the next generation. Cell 144:646–674. doi:10.1016/j.cell.2011.02.013
Liu H, Savaraj N, Priebe W, Lampidis TJ (2002) Hypoxia increases tumor cell sensitivity to glycolytic inhibitors: a strategy for solid tumor therapy (Model C). Biochem Pharmacol 64(12):1745–1751. doi:10.1016/S0006-2952(02)01456-9
Boutrid H, Jockovich ME, Murray TG, Pina Y, Feuer WJ, Lampidis TJ, Cebulla CM (2008) Targeting hypoxia, a novel treatment for advanced retinoblastoma. Invest Ophthalmol Vis Sci 49(7):2799–2805. doi:10.1167/iovs.08-1751
Maschek G, Savaraj N, Priebe W, Braunschweiger P, Hamilton K, Tidmarsh GF, De Young LR, Lampidis TJ (2004) 2-deoxy-d-glucose increases the efficacy of adriamycin and paclitaxel in human osteosarcoma and non-small cell lung cancers in vivo. Cancer Res 64:31–34. doi:10.1158/0008-5472.CAN-03-3294
Robey RB, Hay N (2006) Mitochondrial Hexokinases, novel mediators of the antiapoptotic effects of growth factors and Akt. Oncogene 25:4683–4696. doi:10.1038/sj.onc.1209595
Mathupala SP, Ko YH, Pedersen PL (2006) Hexokinase II: cancer’s double-edged sword acting as both facilitator and gatekeeper of malignancy when bound to mitochondria. Oncogene 25:4777–4786. doi:10.1038/sj.onc.1209603
Pastorino JG, Shulga N, Hoek JB (2002) Mitochondrial binding of hexokinase II inhibits Bax-induced cytochrome c release and apoptosis. J Biol Chem 277(9):7610–7618. doi:10.1074/jbc.M109950200
Kim JW, Dang CV (2005) Multifaceted roles of glycolytic enzymes. Trends Biochem Sci 30:142–150. doi:10.1016/j.tibs.2005.01.005
Majewski N, Nogueira V, Bhaskar P, Coy PE, Skeen JE, Gottlob K, Chandel NS, Thompson CB, Robey RB, Hay N (2004) Hexokinase-mitochondria interaction mediated by Akt is required to inhibit apoptosis in the presence or absence of Bax and Bak. Mol Cell 16:819–830. doi:10.1016/j.molcel.2004.11.014
Ardehali H, Yano Y, Printz RL, Koch S, Whitesell RR, May JM et al (1996) Functional organization of mammalian hexokinase II retention of catalytic and regulatory function in both the NH- and COOH-terminal halves. J Biol Chem 271:1849–1852. doi:10.1074/jbc.271.4.1849
Maher JC, Wangpaichitr M, Savaraj N, Kurtoglu M, Lampidis TJ (2007) Hypoxia-inducible factor-1 confers resistance to the glycolytic inhibitor 2-deoxy-d-glucose. Mol Cancer Ther 6(2):732–741. doi:10.1158/1535-7163.MCT-06-0407
Liang X, Finkel T, Shen D, Yin J, Aszalos A, Gottesman MM (2008) SIRT1 contributes in part to cisplatin resistance in cancer cells by altering mitochondrial metabolism. Mol Cancer Res 6(9):1499–1506. doi:10.1158/1541-7786.MCR-07-2130
Wangpaichitr M, Sullivan EJ, Theodoropoulos G, Wu C, You M, Feun L, Lampidis TJ, Kuo MT, Savaraj N (2012) The relationship of thioredoxin-1 and cisplatin resistance: its impact on ROS and oxidative metabolism in lung cancer cells. Mol Cancer Ther 11:604–615. doi:10.1158/1535-7163.MCT-11-0599
Berezhnoy A, Brenneman R, Bajgelman M, Seales D, Gilboa E (2012) Thermal stability of siRNA modulates aptamer- conjugated siRNA inhibition. Mol Ther Nucleic Acids 16(1):e51. doi:10.1038/mtna.2012.41
Maher JC, Krishan A, Lampidis TJ (2004) Greater cell cycle inhibition and cytotoxicity induced by 2-deoxy-d-glucose in tumor cells treated under hypoxic vs aerobic conditions. Cancer Chemother Pharmacol 53(2):116–122. doi:10.1007/s00280-003-0724-7
Raez LE, Papadopoulos K, Ricart AD, Chiorean EG, Dipaola RS, Stein MN, Rocha Lima CM, Schlesselman JJ, Tolba K, Langmuir VK, Kroll S, Jung DT, Kurtoglu M, Rosenblatt J, Lampidis TJ (2013) A phase I dose-escalation trial of 2-deoxy-d-glucose alone or combined with docetaxel in patients with advanced solid tumors. Cancer Chemother Pharmacol 71(2):523–530. doi:10.1007/s00280-012-2045-1
Wintzell M, Löfstedt L, Johansson J, Pedersen AB, Fuxe J, Shoshan M (2012) Repeated cisplatin treatment can lead to a multiresistant tumor cell population with stem cell features and sensitivity to 3-bromopyruvate. Cancer Bio Ther 13(14):1454–1462. doi:10.4161/cbt.22007
Piña Y, Decatur C, Murray TG, Houston SK, Lopez-Cavalcante M, Hernandez E, Celdran M M, Shah N, Feuer W, Lampidis T (2012) Retinoblastoma treatment: utilization of the glycolytic inhibitor, 2-deoxy-2-fluoro-d-glucose (2-FG), to target the chemoresistant hypoxic regions in LHBETATAG retinal tumors. Invest Ophthalmol Vis Sci 53:996–1002. doi:10.1167/iovs.11-8265
Tajeddine N, Galluzzi L, Kepp O, Hangen E, Morselli E, Senovilla L, Araujo N, Pinna G, Larochette N, Zamzami N, Modjtahedi N, Harel-Bellan A, Kroemer G (2008) Hierarchical involvement of Bak, VDAC1 and Bax in cisplatin-induced cell death. Oncogene 27:4221–4232. doi:10.1038/onc.2008.63
Acknowledgments
This work was supported by the National Cancer Institute Grant #CA37109 to T.J.L. The authors would also like to acknowledge Dr. Niramol Savaraj for her contribution of the SCLC and NSCLC cell lines.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
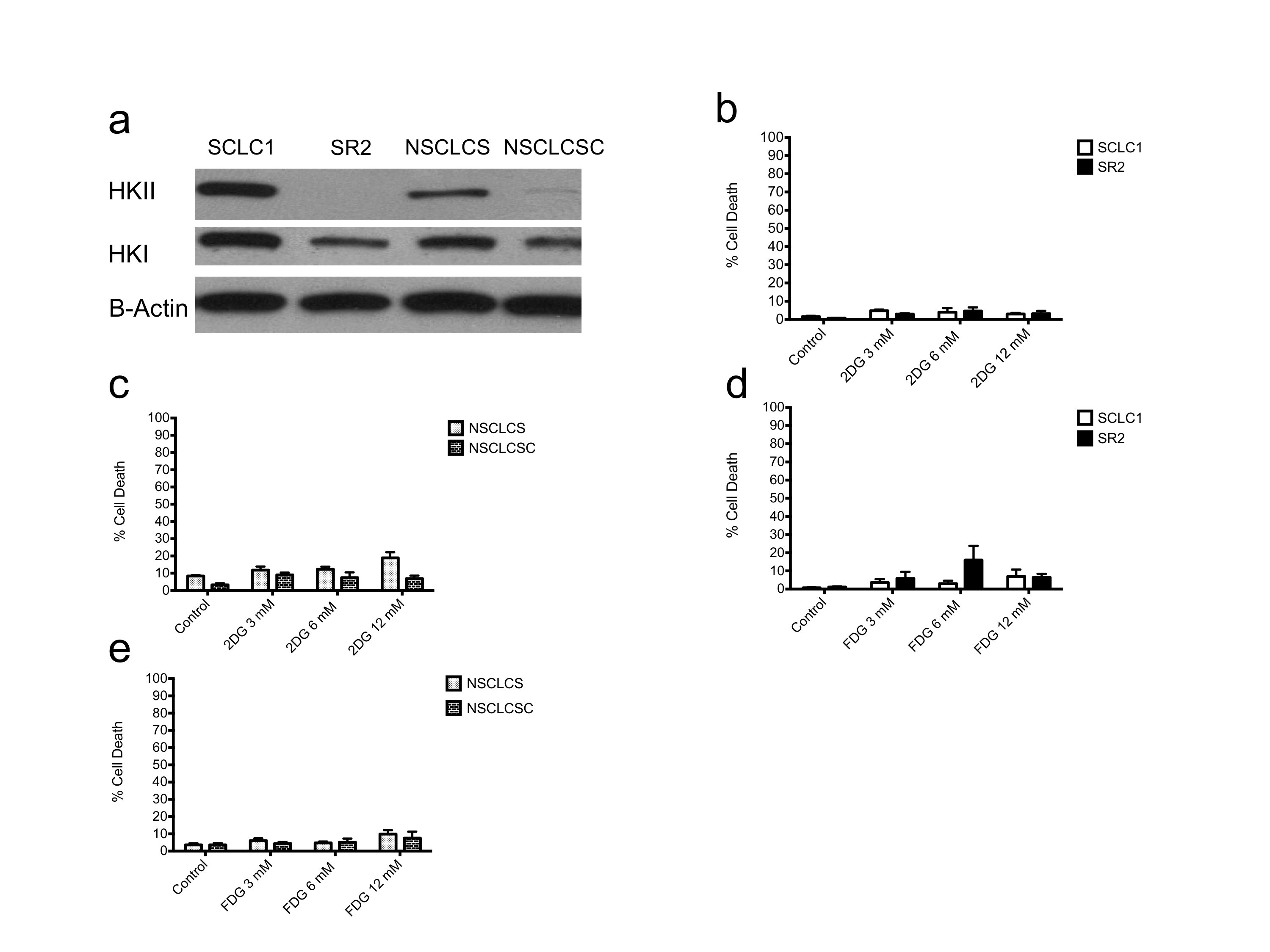
280_2013_2366_MOESM1_ESM.jpg
Oligomycin treatment under normoxia does not increase HK protein levels. a Immunoblot of lung cancer cell lines treated with oligomycin for 24 h. β-actin was used as a loading control. b 24-h 2-DG treatment under normoxia is not cytotoxic in either cisplatin-sensitive SCLC1 or cisplatin-resistant SR2. c 2-DG treatment under normoxia did not induce cell death in NSCLCS or NSCLCSC after 24 h. d 24-h 2-FDG treatment under normoxia is not sufficient to induce cell death in SCLC1 or SR2. e 2-FDG treatment did not induce cell death in cisplatin-sensitive NSCLCS or cisplatin-resistant NSCLCSC. (JPG 373 kb)
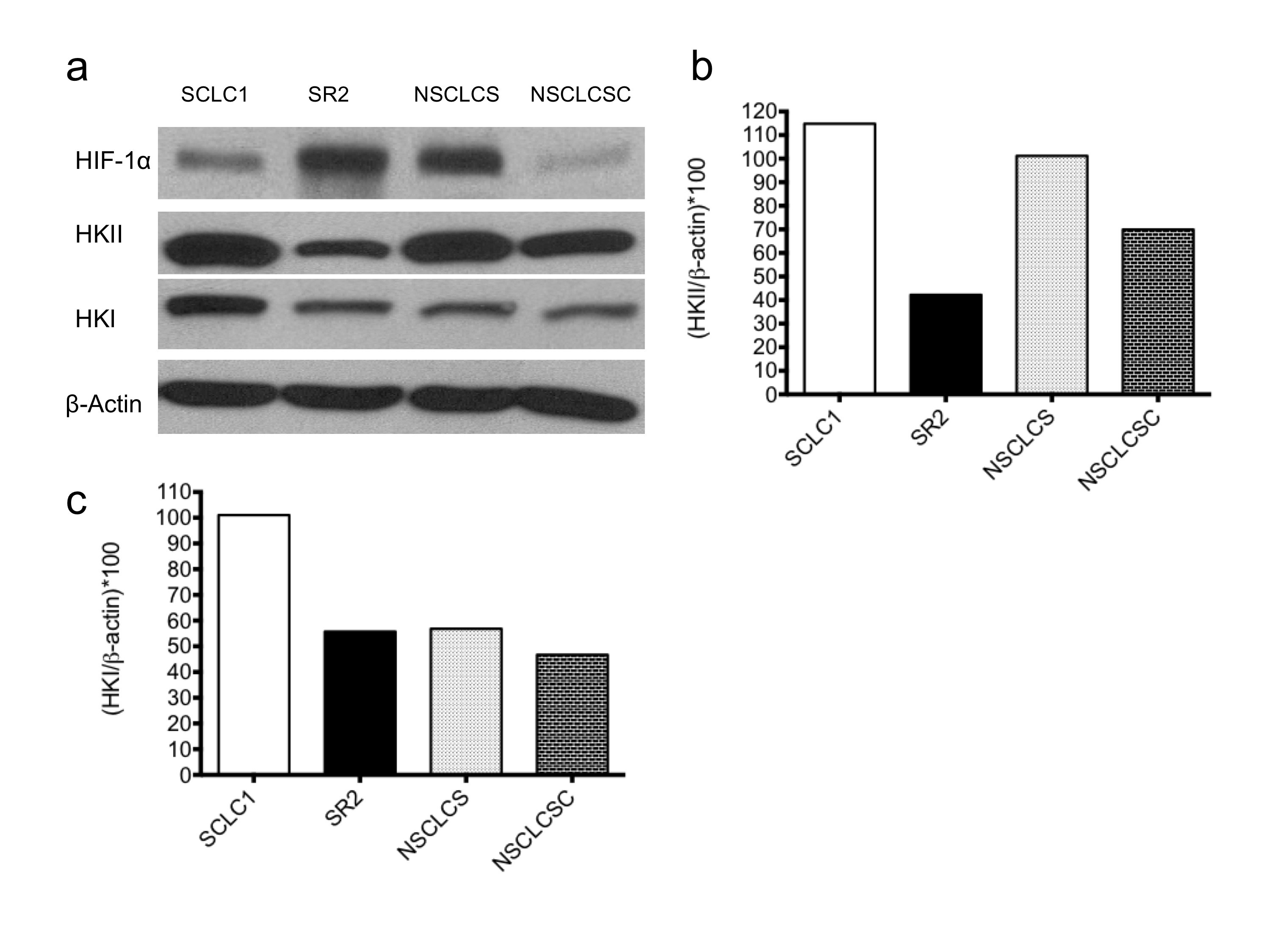
280_2013_2366_MOESM2_ESM.jpg
a Immunoblot probing lung cancer cell lines for HIF-1α, HKII, and HKI levels under 0.5 % O2 hypoxia. β-actin was used as a loading control. b Densitometry of HKII immunoblot for lung cancer cell lines under hypoxia. HKII is lower in cisplatin-resistant SR2 and NSCLCSC than in cisplatin-sensitive parental SCLC1 and NSCLCS, respectively. c Densitometry of HKI immunoblot for lung cancer cell lines under hypoxia. HKI expression is reduced in cisplatin-resistant cell lines compared to cisplatin-sensitive parental cell lines. (JPG 503 kb)

280_2013_2366_MOESM3_ESM.jpg
CPT-1a protein levels are not elevated in cisplatin-resistant NSCLCSC under normoxia compared with cisplatin-sensitive NSCLCS. β-actin was used as a loading control (JPG 148 kb)
Rights and permissions
About this article
Cite this article
Sullivan, E.J., Kurtoglu, M., Brenneman, R. et al. Targeting cisplatin-resistant human tumor cells with metabolic inhibitors. Cancer Chemother Pharmacol 73, 417–427 (2014). https://doi.org/10.1007/s00280-013-2366-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-013-2366-8