Abstract
Background
Irinotecan and cisplatin are one of active regimens for patients with extensive-stage small cell lung cancer (SCLC). To determine the efficacy and toxicity of irinotecan and cisplatin with concurrent split-course thoracic radiotherapy in limited-disease (LD) SCLC, we conducted a phase II study.
Patients and methods
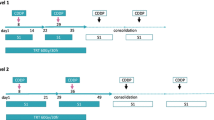
Thirty-four patients fulfilling the following eligibility criteria were enrolled: chemotherapy-naïve, good performance status (PS 0–1), age ≤75, LD-SCLC, and adequate organ function. The patients received irinotecan 40 mg/m2 i.v. on days 1, 8, and 15, and cisplatin 60 mg/m2 i.v. on day 1. Four cycles of chemotherapy were repeated every 4 weeks. Split-course thoracic radiotherapy of once-daily 2 Gy/day commenced on day 2 of each chemotherapy cycle, with 26 and 24 Gy administered in the first and second cycles, respectively.
Results
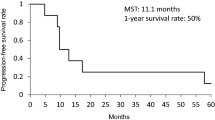
Thirty-four patients were eligible and assessable for response, toxicity, and survival. Patients’ characteristics were as follows: male/female = 29/5; PS 0/1 = 18/16; median age (range) = 67 (50–73); and stage IB/IIA/IIB/IIIA/IIIB = 2/2/3/16/11. The overall response was 100 % (CR 8, PR 26). Grade 4 leukopenia, neutropenia, grade 3–5 pneumonitis, diarrhea, and esophagitis occurred in 24, 38, 6, 3, and 0 %, respectively. There were 2 treatment-related deaths from pneumonitis. The median time to tumor progression was 14.3 months. The median overall survival time and the 2- and 5-year survival rates were 44.5 months, 66.7 and 46.1 %, respectively. No tumor progression was observed in patients with CR.
Conclusion
Irinotecan plus cisplatin with concurrent split-course thoracic radiotherapy was effective and tolerable in untreated LD-SCLC.


Similar content being viewed by others
References
Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM (2010) Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 127:2893–2917
Pignon JP, Arriagada R, Ihde DC, Johnson DH, Perry MC, Souhami RL, Brodin O, Joss RA, Kies MS, Lebeau B, Onoshi T, Osterlind K, Tattersall MHN, Wagner H (1992) A meta-analysis of thoracic radiotherapy for small-cell lung cancer. N Engl J Med 327:1618–1624
Warde P, Payne D (1992) Does thoracic irradiation improve survival and local control in limited-stage small-cell carcinoma of the lung? A meta-analysis. J Clin Oncol 10:890–895
Mascaux C, Paesmans M, Berghmans T, Branle F, Lafitte JJ, Lemaitre F, Meert AP, Vermylen P, Sculier JP, European Lung Cancer Working Party (ELCWP) (2000) A systematic review of the role of etoposide and cisplatin in the chemotherapy of small cell lung cancer with methodology assessment and meta-analysis. Lung Cancer 30:23–36
Curran WJ (2001) Combined-modality therapy for limited-stage small cell lung cancer. Semin Oncol 28:S14–S22
Turrisi AT III, Kim K, Blum R, Sause WT, Livingston RB, Komaki R, Wagner H, Aisner S, Johnson DH (1999) Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med 340:265–271
Takada M, Fukuoka M, Kawahara M, Sugiura T, Yokoyama A, Yokota S, Nishiwaki Y, Watanabe K, Noda K, Tamura T, Fukuda H, Saijo N (2002) Phase III study of concurrent versus sequential thoracic radiotherapy in combination with cisplatin and etoposide for limited-stage small-cell lung cancer: results of the Japan Clinical Oncology Group Study 9104. J Clin Oncol 20:3054–3060
Hsiang YH, Liu LF (1988) Identification of mammalian DNA topoisomerase I as an intracellular target of the anticancer drug camptothecin. Cancer Res 48:1722–1726
Masuda N, Kudoh S, Fukuoka M (1996) Irinotecan (CPT-11): pharmacology and clinical applications. Crit Rev Oncol/Hematol 24:3–26
Fukuda M, Nishio K, Kanzawa F, Ogasawara H, Ishida T, Arioka H, Bojanowski K, Oka M, Saijo N (1996) Synergism between cisplatin and topoisomerase I inhibitors, NB-506 and SN-38, in a human small cell lung cancer cells. Cancer Res 56:789–793
Kanzawa F, Sugimoto Y, Minato K, Kasahara K, Bungo M, Nakagawa K, Fujiwara Y, Liu LF, Saijo N (1990) Establishment of a camptothecin analogue (CPT-11)-resistant cell line of human non-small cell lung cancer: characterization and mechanism of resistance. Cancer Res 50:5919–5924
Kudoh S, Fujiwara Y, Takada Y, Yamamoto H, Kinoshita A, Ariyoshi Y, Furuse K, Fukuoka M (1998) Phase II study of irinotecan combined with cisplatin in patients with previously untreated small-cell lung cancer. West Japan Lung Cancer Group. J Clin Oncol 16:1068–1074
Yokoyama A, Kurita Y, Saijo N, Tamura T, Noda K, Shimokata K, Matsuda T (1998) Dose-finding study of irinotecan and cisplatin plus concurrent radiotherapy for unresectable stage III non-small-cell lung cancer. Br J Cancer 78:257–262
Fukuda M, Soda H, Fukuda M, Kinoshita A, Nakamura Y, Nagashima S, Takatani H, Tsukamoto K, Kohno S, Oka M (2007) Irinotecan and cisplatin with concurrent split-course radiotherapy in locally advanced nonsmall-cell lung cancer: a multiinstitutional phase 2 study. Cancer 110:606–613
Oka M, Fukuda M, Kuba M, Ichiki M, Rikimaru T, Soda H, Tsurutani J, Nakamura Y, Kawabata S, Nakatomi K, Narasaki F, Nagashima S, Takatani H, Fukuda M, Kinoshita A, Kohno S (2002) Phase I study of irinotecan and cisplatin with concurrent split-course radiotherapy in limited-disease small cell lung cancer. Eur J Cancer 38:1998–2004
Therasse P, Arbuch SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst 92:205–216
Simon R (1989) Optional two-stage designs for phase II clinical trials. Control Clin Trials 10:1–10
Kaplan E, Meier P (1958) Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457–481
Noda K, Nishiwaki Y, Kawahara M, Negoro S, Sugiura T, Yokoyama A, Fukuoka M, Mori K, Watanabe K, Tamura T, Yamamoto S, Saijo N (2002) Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med 346:85–91
Hanna N, Bunn PA Jr, Langer C, Einhorn L, Guthrie T Jr, Beck T, Ansari R, Ellis P, Byrne M, Morrison M, Hariharan S, Wang B, Sandler A (2006) Randomized phase III trial comparing irinotecan/cisplatin with etoposide/cisplatin in patients with previously untreated extensive-stage disease small-cell lung cancer. J Clin Oncol 24:2038–2043
Lara PN Jr, Natale R, Crowley J, Lenz HJ, Redman MW, Carleton JE, Jett J, Langer CJ, Kuebler JP, Dakhil SR, Chansky K, Gandara DR (2009) Phase III trial of irinotecan/cisplatin compared with etoposide/cisplatin in extensive-stage small-cell lung cancer: clinical and pharmacogenomics results from SWOG S0124. J Clin Oncol 27:2530–2535
Zatloukal P, Cardenal F, Szczesna A, Gorbunova V, Moiseyenko V, Zhang X, Cisar L, Soria JC, Domine M, Thomas M (2010) A multicenter international randomized phase III study comparing cisplatin in combination with irinotecan or etoposide in previously untreated small-cell lung cancer patients with extensive disease. Ann Oncol 21:1810–1816
Jiang J, Liang X, Zhou X, Huang L, Huang R, Chu Z, Zhan Q (2010) A meta-analysis of randomized controlled trials comparing irinotecan/platinum with etoposide/platinum in patients with previously untreated extensive-stage small cell lung cancer. J Thorac Oncol 5:867–873
Langer CJ, Somer R, Litwin S, Feigenberg S, Movsas B, Malale C, Sherman E, Millenson M, Nicoloau N, Huang C, Treat J (2007) Phase I study of radical thoracic radiation, weekly irinotecan, and cisplatin in locally advanced non-small cell lung carcinoma. J Thorac Oncol 2:203–209
Han JY, Cho KH, Lee DH, Kim HY, Kim EA, Lee SY, Lee JS (2005) Phase II study of irinotecan plus cisplatin induction followed by concurrent twice-daily thoracic irradiation with etoposide plus cisplatin chemotherapy for limited-disease small-cell lung cancer. J Clin Oncol 23:3488–3494
Kubota K, Nishiwaki Y, Sugiura T, Noda K, Mori K, Kawahara M, Negoro S, Watanabe K, Imamura F, Tamura T, Saijo N (2005) Pilot study of concurrent etoposide and cisplatin plus accelerated hyperfractionated thoracic radiotherapy followed by irinotecan and cisplatin for limited-stage small cell lung cancer: Japan Clinical Oncology Group 9903. Clin Cancer Res 11:5534–5538
Saito H, Takada Y, Ichinose Y, Eguchi K, Kudoh S, Matsui K, Nakagawa K, Takada M, Negoro S, Tamura K, Ando M, Tada T, Fukuoka M, West Japan Thoracic Oncology Group 9902 (2006) Phase II study of etoposide and cisplatin with concurrent twice-daily thoracic radiotherapy followed by irinotecan and cisplatin in patients with limited-disease small-cell lung cancer: West Japan Thoracic Oncology Group 9902. J Clin Oncol 24:5247–5252
Sohn JH, Moon YW, Lee CG, Kim GE, Chung KY, Chang J, Kim SK, Kim YS, Choi BW, Choi HJ, Kim JH (2007) Phase II trial of irinotecan and cisplatin with early concurrent radiotherapy in limited-disease small-cell lung cancer. Cancer 109:1845–1850
Pijls-Johannesma M, De Ruysscher D, Vansteenkiste J, Kester A, Rutten K, Lambin P (2007) Timing of chest radiotherapy in patients with limited stage small cell lung cancer: a systematic review and meta-analysis of randomized controlled trials. Cancer Treat Rev 33:461–473
Blackstock AW, Bogart JA, Matthews C, Lovato JF, McCoy T, Livengood K, Ho C, White D, Atkins JN, Miller AA (2005) Split-course versus continuous thoracic radiation therapy for limited-stage small-cell lung cancer: final report of a randomized phase III trial. Clin Lung Cancer 6:287–292
Gielda BT, Marsh JC, Zusag TW, Faber LP, Liptay M, Basu S, Warren WH, Fidler MJ, Batus M, Abrams RA, Bonomi P (2011) Split-course chemoradiotherapy for locally advanced non-small cell lung cancer. A single-institution experience of 144 patients. J Thorac Oncol 6:1079–1086
Sorensen M, Pijls-Johannesma M, Felip E (2010) Small-cell lung cancer: ESMO clinical guidelines for diagnosis, treatment and follow-up. Ann Oncol 21:v120–v125
Miller KL, Marks LB, Sibley GS, Clough RW, Garst JL, Crawford J, Shafman TD (2003) Routine use of approximately 60 Gy once-daily thoracic irradiation for patients with limited-stage small-cell lung cancer. Int J Radiat Oncol Biol Phys 56:355–359
Schild SE, Bonner JA, Shanahan TG, Brooks BJ, Marks RS, Geyer SM, Hillman SL, Farr GH Jr, Tazelaar HD, Krook JE, Geoffroy FJ, Salim M, Arusell RM, Mailliard JA, Schaefer PL, Jett JR (2004) Long-term results of a phase III trial comparing once-daily radiotherapy with twice-daily radiotherapy in limited-stage small-cell lung cancer. Int J Radiat Oncol Biol Phys 59:943–951
Ohe Y, Yamamoto S, Suzuki K, Hojo F, Kakinuma R, Matsumoto T, Ohmatsu H, Nishiwaki Y (2001) Risk factors of treatment-related death in chemotherapy and thoracic radiotherapy for lung cancer. Eur J Cancer 37:54–63
Conflict of interest
The authors report no potential conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fukuda, M., Nakamura, Y., Kinoshita, A. et al. Phase II study of irinotecan and cisplatin with concurrent split-course radiotherapy in limited-disease small cell lung cancer. Cancer Chemother Pharmacol 70, 645–651 (2012). https://doi.org/10.1007/s00280-012-1952-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-012-1952-5