Abstract
Purpose
The prognosis of patients with unresectable M0 gastric cancer remains very poor. We performed a phase II trial to explore the efficacy and toxicity of induction irinotecan-cisplatin (IC) followed by concurrent irinotecan-cisplatin and radiotherapy (IC/RT) in this setting.
Methods and materials
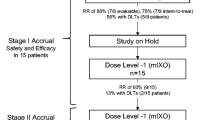
Patients with unresectable M0 gastric (GC) or oesophageal-gastric junction (EGJC) adenocarcinomas were treated with two courses of IC (irinotecan, 65 mg/m2; cisplatin, 30 mg/m2 on days 1 and 8 every 21 days) followed by IC/RT (daily radiotherapy—45 Gy—with concurrent IC: irinotecan, 65 mg/m2, and cisplatin, 30 mg/m2, on days 1, 8, 15, and 22). Resectability was reassessed after this treatment, and surgical resection was performed if feasible. The primary endpoint was the R0 resection rate after induction treatment.
Results
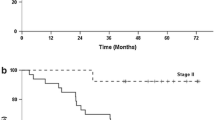
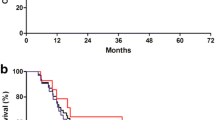
Seventeen patients were included in the study (EGJC: 6; GC: 11). An R0 resection was achieved in only 5 patients (29%), and according to the design of the trial (Simon’s optimal two-stage) accrual of patients was terminated after the first stage. No patient died during IC, whereas 3 patients (24%) died during IC/RT and one of 5 resected patients (20%) died during the first 30 days after resection. The median survival was 10.5 months, and the actuarial 2-year survival rate was 27%.
Conclusions
Induction IC followed by IC/RT showed poor efficacy and significant toxicity in patients with unresectable GC/EGJC.



Similar content being viewed by others
References
Ferlay J, Autier P, Boniol M et al (2007) Estimates of the cancer incidence and mortality in Europe in 2006. Ann Oncol 18:581–592
Brennan MF, Karpeh MS Jr (1996) Surgery for gastric cancer: the American view. Semin Oncol 23:352
Chau I, Norman AR, Cunningham D et al (2009) The impact of primary tumour origins in patients with advanced oesophageal, oesophago-gastric junction and gastric adenocarcinoma—individual patient data from 1775 patients in four randomised controlled trials. Ann Oncol 20(5):885–891
Wilke H, Preusser P, Fink U et al (1989) Preoperative chemotherapy in locally advanced and nonresectable gastric cancer: a phase II study with etoposide, doxorubicin, and cisplatin. J Clin Oncol 7(9):1318–1326
Plukker JT, Mulder NH, Sleijfer DT et al (1991) Chemotherapy and surgery for locally advanced cancer of the cardia and fundus: phase II study with methotrexate and 5-fluorouracil. Br J Surg 78(8):955–958
Cascinu S, Scartozzi M, Labianca R et al (2004) High curative resection rate with weekly cisplatin, 5-fluorouracil, epidoxorubicin, 6S-leucovorin, glutathione, and filgastrim in patients with locally advanced, unresectable gastric cancer: a report from the Italian Group for the Study of Digestive Tract Cancer (GISCAD). Br J Cancer 90(8):1521–1525
Shim S, Chang H, Ryu M et al (2007) Phase II study of neoadjuvant chemotherapy with docetaxel, capecitabine and cisplatin (DXP) in patients with locally advanced unresectable or intra-abdominal metastatic gastric cancer. Gastrointest Cancers Symp, Abstract No: 65
Stahl M, Walz MK, Stuschke M et al (2009) Phase III comparison of preoperative chemotherapy compared with chemoradiotherapy in patients with locally advanced adenocarcinoma of the esophagogastric junction. J Clin Oncol 27(6):851–856
Ajani JA (2005) Evolving chemotherapy for advanced gastric cancer. Oncologist 10(Suppl 3):49–58
Ilson DH, Saltz L, Enzinger P et al (1999) Phase II trial of weekly irinotecan plus cisplatin in advanced esophageal cancer. J Clin Oncol 17(10):3270–3275
Ilson DH, Bains M, Kelsen DP et al (2003) Phase I trial of escalating-dose irinotecan given weekly with cisplatin and concurrent radiotherapy in locally advanced esophageal cancer. J Clin Oncol 21(15):2926–2932
Mandard AM, Dalibard F, Mandard JC et al (1994) Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Cancer 73:2680–2686
Simon R (1989) Optimal two-stage designs for phase II trials. Control Clin Trials 10:1–10
Kaplan EL, Meier P (1958) Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457–481
Ajani JA, Winter K, Okawara GS et al (2006) Phase II trial of preoperative chemoradiation in patients with localized gastric adenocarcinoma (RTOG 9904): quality of combined modality therapy and pathological response. J Clin Oncol 24:3953–3958
Pozzo C, Barone C, Szanto J et al (2004) Irinotecan in combination with 5-fluorouracil and folinic acid or with cisplatin in patients with advanced gastric or esophageal-gastric junction adenocarcinoma: results of a randomized phase II study. Ann Oncol 15:1773–1781
Dank M, Zaluski J, Barone C et al (2005) Randomized phase 3 trial of irinotecan (CPT-11) + 5FU/folinic acid (FA) vs CDDP + 5FU in 1st-line advanced gastric cancer patients. J Clin Oncol 23(suppl):308s Abstract No 4003
Rivera F, Galán M, Tabernero J et al (2009) Phase II trial of preoperative irinotecan-cisplatin followed by concurrent irinotecan-cisplatin and radiotherapy for resectable locally advanced gastric and esophagogastric junction adenocarcinoma. Int J Radiat Oncol Biol Phys [Epub ahead of print]
Ajani JA, Mansfield PF, Janjan N et al (2004) Multi-institutional trial of preoperative chemoradiotherapy in patients with potentially resectable gastric carcinoma. J Clin Oncol 22:2774–2780
Ajani JA, Mansfield PF, Crane CH et al (2005) Paclitaxel-based chemoradiotherapy in localized gastric carcinoma: degree of pathologic response and not clinical parameters dictated patient outcome. J Clin Oncol 23:1237–1244
Van Cutsem E, Kang Y, Chung H et al (2009) Efficacy results from the ToGA trial: a phase III study of trastuzumab added to standard chemotherapy (CT) in first-line human epidermal growth factor receptor 2 (HER2)-positive advanced gastric cancer (GC). J Clin Oncol 27(suppl):18s Abstract No LBA4509
Acknowledgments
We thank the patients and the medical and nursing staff of all the participating institutions. We also thank David Asensio, Julita Ocaña, Matilde Salcedo, Noelia Vega, Yolanda Blanco and Inmaculada Ruiz de Mena for their helpful comments on the manuscript. This work was supported by an unrestricted grant from Laboratorios Almirall, Barcelona, Spain.
Conflict of interest
Fernando Rivera served on the advisory board and received travel and research grants from Roche, Sanofi –Aventis and Merck-Serono. Maica Galán served on the advisory board for Roche. No conflicts of interest pertain to the remaining authors.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Rivera, F., Galán, M., Tabernero, J. et al. Phase II trial of induction irinotecan-cisplatin followed by concurrent irinotecan-cisplatin and radiotherapy for unresectable, locally advanced gastric and oesophageal-gastric junction adenocarcinoma. Cancer Chemother Pharmacol 67, 75–82 (2011). https://doi.org/10.1007/s00280-010-1285-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-010-1285-1