Abstract
Purpose
The objective of the present study was to characterize the population pharmacokinetics of melphalan infused over a 24-h period in patients with advanced malignancies.
Methods
Enrolled in the study were 64 patients (144 courses). The population pharmacokinetic analysis was performed using NONMEM through the graphical interface Visual-NM. Population characteristics were computed from an initial group of 43 patients (99 courses), and 21 additional patients (45 courses) were used for model validation. With the use of a one-compartment model, the influence of demographic and biological characteristics was examined. The basic parameters were total clearance (CL) and volume of distribution (V). The interoccasion variability was taken into account in the model. The drug exposure was estimated for each patient and correlated with markers of efficacy and toxicity.
Results
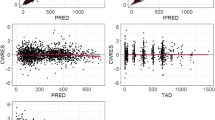
Data analysis was performed using a three-step approach. In step 2, a close relationship was found between creatinine clearance, gender and melphalan CL. The inclusion of this second stage model significantly improved the fit. Melphalan CL was higher in male patients (14.3±4.5 l/h per m2) than in female patients (12.3±4.5 l/h per m2). CL was also reduced somewhat in patients with decreased creatinine clearance. Large interindividual variability in pharmacokinetic parameters occurred (CL varied from 4.4 to 30.6 l/h per m2). The percentage intrapatient variability in clearance between courses was 25.4%. For determining melphalan AUC in clinical routine from one sample drawn at steady state, Bayesian methodology allowed a more accurate estimation of CL than the classical formula. Neutropenia and thrombocytopenia were the main haematological toxicities encountered; grade 4 was observed in 34 and 22 courses over a total of 144 courses, respectively. No significant relationship between AUC and haematological toxicity was found. In patients with prostatic cancer a weak relationship was observed between the decrease in PSA levels and AUC (P=0.0457), while in patients with ovarian cancer no relationship was found between AUC and CA125 levels.
Conclusion
The population pharmacokinetic approach developed in this study should allow dosage to be individualized in order to decrease toxicity while maintaining good efficacy.





Similar content being viewed by others
References
Adair CG, Bridges JM, Desai ZR (1986) Renal function in the elimination of oral melphalan in patients with multiple myeloma. Cancer Chemother Pharmacol 17:185
Ardiet C, Tranchand B, Biron P, Rebattu P, Philip T (1986) Pharmacokinetics of high-dose intravenous melphalan in children and adults with forced diuresis. Report in 26 cases. Cancer Chemother Pharmacol 16:300
Beal SL, Sheiner LB (1992) NONMEM user’s guide. University of California at San Francisco, San Francisco, CA
Bergel F, Stock JA (1953) Cytotoxic alpha amino acids and endopeptidase. Br Emp Cancer Annu 31:6
Bosanquet AG, Bird MC (1988) Degradation of melphalan in vitro: rationale for the use of continuous exposure in chemosensitivity assays. Cancer Chemother Pharmacol 21:211
Bosanquet AG, Gilby ED (1982) Pharmacokinetics of oral and intravenous melphalan during routine treatment of multiple myeloma. Eur J Cancer Clin Oncol 18:355
Bozdogan H (1987) Model selection and Akaike’s information criteria (AIC): the general theory and its analytical extension. Psychometrika 52:345
Cockcroft DW, Gault MH (1976) Prediction of creatinine clearance from serum creatinine. Nephron 16:31
Cornwell GG III, Pajak TF, McIntyre OR, Kochwa S, Dosik H (1982) Influence of renal failure on myelosuppressive effects of melphalan: Cancer and Leukemia Group B experience. Cancer Treat Rep 66:475
Gouyette A, Hartmann O, Pico JL (1986) Pharmacokinetics of high-dose melphalan in children and adults. Cancer Chemother Pharmacol 16:184
Green JA, Vistica DT, Young RC, Hamilton TC, Rogan AM, Ozols RF (1984) Potentiation of melphalan cytotoxicity in human ovarian cancer cell lines by glutathione depletion. Cancer Res 44:5427
Hersh MR, Ludden TM, Kuhn JG, Knight WA III (1983) Pharmacokinetics of high dose melphalan. Invest New Drugs 1:331
Karlsson MO, Sheiner LB (1993) The importance of modeling interoccasion variability in population pharmacokinetic analyses. J Pharmacokinet Biopharm 21:735
Ninane J, Baurain R, de Selys A, Trouet A, Cornu G (1985) High dose melphalan in children with advanced malignant disease. A pharmacokinetic study. Cancer Chemother Pharmacol 15:263
Ossenkoppele GJ (1997) Treatment of multiple myeloma in elderly patients. New developments. Drugs Aging 11:152
Pinguet F, Martel P, Rouanet P, Fabbro M, Astre C (1994) Effect of sodium chloride concentration and temperature on melphalan stability during storage and use. Am J Hosp Pharm 51:2701
Pinguet F, Joulia JM, Martel P, Grosse PY, Astre C, Bressolle F (1996) High-performance liquid chromatographic assay for melphalan in human plasma. Application to pharmacokinetic studies. J Chromatogr B Biomed Appl 686:43
Pinguet F, Martel P, Fabbro M, Petit I, Canal P, Culine S, Astre C, Bressolle F (1997) Pharmacokinetics of high-dose intravenous melphalan in patients undergoing peripheral blood hematopoietic progenitor-cell transplantation. Anticancer Res 17:605
Pinguet F, Bressolle F, Culine S, Fabbro M, Astre C, Chevillard C (1999) Influence of the schedule of exposure on the cytotoxic effect of melphalan on human 8226 and A2780 cells. Eur J Cancer 35:1402
Pinguet F, Culine S, Bressolle F, Astre C, Serre MP, Chevillard C, Fabbro M (2000) A phase I and pharmacokinetic study of melphalan using a 24-hour continuous infusion in patients with advanced malignancies. Clin Cancer Res 6:57
RDPP (1998) Visual-NM program, version 5.1. RDPP, Montpellier, France
Samuels BL, Bitran JD (1995) High-dose intravenous melphalan: a review. J Clin Oncol 13:1786
Sarosy G, Leyland-Jones B, Soochan P, Cheson BD (1988) The systemic administration of intravenous melphalan. J Clin Oncol 6:1768
Sheiner BL, Beal SL (1981) Evaluation of methods for estimating population pharmacokinetic parameters. II. Biexponential model and experimental pharmacokinetic data. J Pharmacokinet Biopharm 9:635
Sheiner LB, Beal SL (1981) Some suggestions for measuring predictive performance. J Pharmacokinet Biopharm 9:503
Taha IA, Ahmad RA, Rogers DW, Pritchard J, Rogers HJ (1983) Pharmacokinetics of melphalan in children following high-dose intravenous injection. Cancer Chemother Pharmacol 10:212
Tattersall MH, Jarman M, Newlands ES, Holyhead L, Milstead RA, Weinberg A (1978) Pharmaco-kinetics of melphalan following oral or intravenous administration in patients with malignant disease. Eur J Cancer 14:507
Thigpen T, Vance R, Puneky L, Khansur T (1994) Chemotherapy in advanced ovarian carcinoma: current standards of care based on randomized trials. Gynecol Oncol 55: S97
Tranchand B, Ploin YD, Minuit MP, Sapet C, Biron P, Philip T, Ardiet C (1989) High-dose melphalan dosage adjustment: possibility of using a test-dose. Cancer Chemother Pharmacol 23:95
Woodhouse KW, Hamilton P, Lennard A, Rawlins MD (1983) The pharmacokinetics of melphalan in patients with multiple myeloma: an intravenous/oral study using a conventional dose regimen. Eur J Clin Pharmacol 24:283
Acknowledgements
The authors gratefully acknowledge support for this work from the “Ligue Nationale de Lutte contre le Cancer”, Montpellier, France. We would also like to thank Mme. F. Malosse for her technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mougenot, P., Pinguet, F., Fabbro, M. et al. Population pharmacokinetics of melphalan, infused over a 24-hour period, in patients with advanced malignancies. Cancer Chemother Pharmacol 53, 503–512 (2004). https://doi.org/10.1007/s00280-003-0761-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-003-0761-2