Abstract
Purpose
The aim of this study was to determine the behavior of docetaxel (DTX) in ovarian cancer cells resistant to paclitaxel (PTX).
Methods
We used human ovarian adenocarcinoma cell lines KF, KFTx (PTX-resistant KF), SK-OV-3, and HAC-2. The sensitivity of the cells to PTX or DTX was determined by the MTT assay. Cellular accumulation of PTX and DTX was measured by high-performance liquid chromatography. mRNA of MDR-1 was detected by RT-PCR. Cell cycle distribution was determined by flow cytometry after exposure to the IC50 of each drug. Bcl-2 phosphorylation was determined by Western blot analysis. Activity for tubulin polymerization of each drug was examined by a β-tubulin polymerization assay.
Results
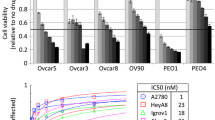
KFTx cells had a 5.5-fold greater resistance to PTX and a 7.3-fold greater resistance to DTX than KF cells, indicating that KFTx cells had acquired cross-resistance to DTX. SK-OV-3 cells were sensitive and HAC-2 cells were resistant to both PTX and DTX. The gene expression of MDR-1 increased after exposure to DTX in KF and KFTx cells. Residual cellular accumulation of PTX and DTX was significantly lower in KFTx cells than in KF cells. In contrast, MDR-1 expression was not detected in SK-OV-3 and HAC-2 cells. Flow cytometric analysis indicated no differences in alterations of cell cycle distribution following exposure to the two drugs. Bcl-2 phosphorylation occurred after exposure to DTX at a concentration equivalent to the clinical dose, but did not occur after exposure to PTX in KFTx cells. In HAC-2 cells, Bcl-2 phosphorylation was not detected after exposure to DTX or PTX at concentrations equivalent to the clinical doses. DTX showed greater tubulin polymerization activity than PTX in KFTx cells. β-tubulin polymerization did not correlate with the concentration of PTX or DTX, suggesting that alteration in the tubulin reaction might contribute to the resistance in HAC-2 cells.
Conclusions
The present study suggests that the mechanisms involved in cytotoxicity of and resistance to PTX and DTX do not differ, but DTX has a greater cytotoxic potential in PTX-resistant cells with an efflux system.





Similar content being viewed by others
References
Basu A, Haldar S (1998) Microtubule-damaging drugs triggered bcl2 phosphorylation-requirement of phosphorylation on both serine-70 and serine-87 residues of bcl2 protein. Int J Oncol 13:659
Benjapibal M, Kudelka AP, Vasuratna A, Edwards CL, Verschraegen CF, Valero V, Vadhan-Raj S, Kavanagh JJ (1998) Docetaxel and cyclophosphamide induced remission in platinum and paclitaxel refractory ovarian cancer. Anticancer Drugs 9:577
Blagosklonny MV, Giannakakou P, El-Deiry WS, Kingston DG, Higgs PI, Neckers L, Fojo T (1997) Raf-1/bcl-2 phosphorylation: a step from microtubule damage to cell death. Cancer Res 57:130
Britten RA, Klein K (2000) Differential impact of Raf-1 kinase activity on tumor cell resistance to paclitaxel and docetaxel. Anticancer Drugs 11:439
Chomcynski P, Sacchi N (1987) Single-step method of DNA isolation by acid guanidinium thiocyanate-chloroform extraction. Anal Biochem 162:156
Christen RD, Jekunen AP, Jones JA, Thiebaut F, Shalinsky DR, Howell SB (1993) In vitro modulation of cisplatin accumulation in human ovarian carcinoma cells by pharmacologic alteration of microtubules. J Clin Invest 92:431
Cortes JE, Pazdur R (1995) Docetaxel. J Clin Oncol 13:2643
Crown J, O’Leary M (2000) The taxanes: an update. Lancet 355:1176
Dumontet C, Sikic BI (1999) Mechanisms of action of and resistance to antitubulin agents: microtubule dynamics, drug transport, and cell death. J Clin Oncol 17:1061
Engblom P, Rantanen V, Kulmala J, Heiskanen J, Grenman S (1997) Taxane sensitivity of ovarian carcinoma in vitro. Anticancer Res 17:2475
Giannakakou P, Poy G, Zhan Z, Knutsen T, Blagosklonny MV, Fojo T (2000) Paclitaxel selects for mutant or pseudo-null p53 in drug resistance associated with tubulin mutations in human cancer. Oncogene 19:3078
Hortobagyi GN (1999) Recent progress in the clinical development of docetaxel (Taxotere). Semin Oncol 26 [3 Suppl 9]:32
Horwitz SB (1992) Mechanism of action of taxol. Trends Pharmacol Sci 13:134
Huizing MT, Keung AC, Rosing H, van der Kuij V, ten Bokkel Huinink, WW Mandjes IM, Dubbelman AC, Pinedo HM, Beijnen JH (1993) Pharmacokinetics of paclitaxel and metabolites in a randomized comparative study in platinum-pretreated ovarian cancer patients. J Clin Oncol 11:2127
Kamazawa S, Kigawa J, Minagawa Y, Itamochi H, Shimada M, Takahashi M, Sato S, Akeshima R, Terakawa N (2000) Cellular efflux pump and interaction between cisplatin and paclitaxel in ovarian cancer cells. Oncology 59:329
Kamazawa S, Kigawa J, Kanamori Y, Itamochi H, Sato S, Iba T, Terakawa N (2002) Multidrug resistance gene-1 is a useful predictor of paclitaxel-based chemotherapy for patients with ovarian cancer. Gynecol Oncol 86:171
Kaye SB, Piccart M, Aapro M, Francis P, Kavanagh J (1997) Phase II trials of docetaxel (Taxotere) in advanced ovarian cancer—an updated overview. Eur J Cancer 33:2167
Longnecker SM, Donehower RC, Cates AE, Chen TL, Brundrett RB, Grochow LB, Ettinger DS, Colvin M (1987) High performance liquid chromatographic assay for taxol in human plasma and uterine and pharmacokinetics in phase I trial. Cancer Treat Rep 71:56
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55
Parness J, Horwitz SB (1981) Taxol binds to polymerized tubulin in vitro. J Cell Biol 91:479
Piccart MJ, Gore M, Ten Bokkel Huinink W, van Oosterom A, Verweij J, Wanders J, Franklin H, Bayssas M, Kaye S (1995) Docetaxel: an active new drug for treatment of advanced epithelial ovarian cancer. J Natl Cancer Inst 87:676
Poruchynsky MS, Wang EE, Rudin CM, Blagosklonny MV, Fojo T (1998) Bcl-xL is phosphorylated in malignant cells following microtubule disruption. Cancer Res 58:3331
Valero V (1996) Treatment of patients resistant to paclitaxel therapy. Anticancer Drugs 7 [Suppl 2]:17
Veitia R, Bissery MC, Martinez C, Fellous A (1998) Tau expression in model adenocarcinomas correlates with docetaxel sensitivity in tumour-bearing mice. Br J Cancer 78:87
Verschraegen CF, Sittisomwong T, Kudelka AP, Guedes ED, Steger M, Nelson-Taylor T, Vincent M, Rogers R, Atkinson EN, Kavanagh JJ (2000) Docetaxel for patients with paclitaxel-resistant Mullerian carcinoma. J Clin Oncol 18:2733
Verweiji J, Claveli M, Chevalier B (1994) Paclitaxel (Taxol) and docetaxel (Taxotere): not simply two of a kind. Ann Oncol 5:495
Wang S, Wang Z, Boise L, Dent P, Grant S (1999) Loss of the bcl-2 phosphorylation loop domain increases resistance of human leukemia cells (U937) to paclitaxel-mediated mitochondrial dysfunction and apoptosis. Mol Cell Biol 19:8469
Yamamoto K, Ichijo H, Korsmeyer SJ (1999) BCL-2 is phosphorylated and inactivated by an ASK1/Jun N-terminal protein kinase pathway normally activated at G(2)/M. Biochem Biophys Res Commun 259:67
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sato, S., Kigawa, J., Kanamori, Y. et al. Activity of docetaxel in paclitaxel-resistant ovarian cancer cells. Cancer Chemother Pharmacol 53, 247–252 (2004). https://doi.org/10.1007/s00280-003-0714-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-003-0714-9