Abstract
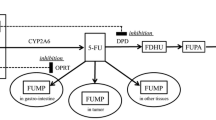
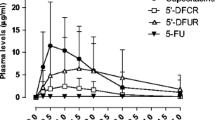
S-1 is an oral formulation of ftorafur (FT), oxonic acid and 5-chloro-2,4-dihydroxypyridine (CDHP) at a molar ratio of 1:0.4:1. FT is a 5-fluorouracil (5-FU) prodrug, CDHP is a dihydropyrimidine dehydrogenase (DPD) inhibitor and oxonic acid is an inhibitor of 5-FU phosphoribosylation in the gastrointestinal mucosa and was included to prevent gastrointestinal toxicity. We determined the pharmacokinetics of S-1 in 28 patients at doses of 25, 35, 40 and 45 mg/m2. The plasma Cmax values of FT, 5-FU, oxonic acid and CDHP increased dose-dependently and after 1–2 h were in the ranges 5.8–13 μM, 0.4–2.4 μM, 0.026–1.337 μM, and 1.1–3.6 μM, respectively. Uracil levels, indicative of DPD inhibition, also increased dose-dependently from basal levels of 0.03–0.25 μM to 3.6–9.4 μM after 2–4 h, and 0.09–0.9 μM was still present after 24 h. The pharmacokinetics of CDHP and uracil were linear over the dose range. The areas under the plasma concentration curves (AUC) for CDHP and uracil were in the ranges 418–1735 and 2281–8627 μmol·min/l, respectively. The t1/2 values were in the ranges 213–692 and 216–354 min, respectively. Cumulative urinary excretion of FT was predominantly as 5-FU and was 2.2–11.9%; the urinary excretion of both fluoro-β-alanine and uracil was generally maximal between 6 and 18 h. During 28-day courses with twice-daily S-1 administration, 5-FU and uracil generally increased. Before each intake of S-1, 5-FU varied between 0.5 and 1 μM and uracil was in the micromolar range (up to 7 μM), indicating that effective DPD inhibition was maintained during the course. In a biopsy of an esophageal adenocarcinoma metastasis that had regressed, thymidylate synthase, the target of 5-FU, was inhibited 50%, but increased four- to tenfold after relapse in subsequent biopsies. In conclusion, oral S-1 administration resulted in prolonged exposure to micromolar 5-FU concentrations due to DPD inhibition, and the decrease in uracil levels after 6 h followed the pattern of CDHP and indicates reversible DPD inhibition.








Similar content being viewed by others
References
Peters GJ, Köhne CH (1999) Fluoropyrimidines as antifolate drugs. In: Jackman AL (ed) Antifolate drugs in cancer therapy. Humana Press, Totowa, pp 101–145
Pizzorno G, Sun Z, Handschumacher RE (1995) Aberrant cell cycle inhibition pattern in human colon carcinoma cell lines after exposure to 5-fluorouracil. Biochem Pharmacol 49:553–557
Backus HHJ, Pinedo HM, Wouters D, Kuiper CM, Jansen G, Van Groeningen CJ, Peters GJ (2000) Differences in the induction of DNA damage, cell cycle arrest, and cell death by 5-fluorouracil and antifolates. Oncol Res 12:231–239
Drewinko B, Yang LY (1985) Cellular basis for the inefficacy of 5-FU in human colon carcinoma. Cancer Treat Rep 69:1391–1398
Peters GJ, Laurensse E, Leyva A, Pinedo HM (1987) Purine nucleosides as cell-specific modulators of 5-fluorouracil metabolism and cytotoxicity. Eur J Cancer Clin Oncol 23:1869–1881
Meta-Analysis Group in Cancer (1998) Efficacy of intravenous continuous infusion of fluorouracil compared with bolus administration in advanced colorectal cancer. J Clin Oncol 16:301–308
Myers CE (1981) The pharmacology of fluoropyrimidines. Pharmacol Rev 33:1–15
El Sayed YM, Sadée W (1983) The fluoropyrimidines. In: Ames MM, Powis G, Kovach JS (eds) Pharmacokinetics of anticancer agents in humans. Elsevier Science Publishers, Amsterdam, pp 209–227
Abernethy DR, Alper JC, Wiemann MC, McDonald CJ, Calabresi P (1989) Oral 5-fluorouracil in psoriasis: pharmacokinetic-pharmacodynamic relationships. Pharmacology 39:78–88
Collins JM, Dedrick RL, King FG, Speyer JL, Myers CE (1980) Non-linear pharmacokinetic models for 5-fluorouracil in man. Clin Pharmacol Ther 28:235–246
Diasio RB (2000) Oral DPD-inhibitory fluoropyrimidine drugs. Oncology 14:19–23
Peters GJ, Ackland SP (1996) New antimetabolites in preclinical and clinical development. Expert Opinion Invest Drugs 5:637–679
Hoff PM, Pazdur R, Benner SE, et al (1998) UFT and leucovorin: a review of its clinical development and therapeutic potential in the oral treatment of cancer. Anticancer Drugs 9:479–490
Shirasaka T, Nakano K, Takechi T, Satake H, Uchida J, Fujioka A, Saito H, Okabe H, Oyama K, Takeda S, Unemi N, Fukushima M (1996) Antitumor activity of 1 M FT-0.4 M 5-chloro-2,4-dihydroxypyridine-1 M potassium oxonate (S-1) against human colon carcinoma orthotopically implanted into nude rats. Cancer Res 56:2602–2606
Schilsky RL, Hohneker J, Ratain MJ, Janisch L, Smetzer L, Lucas VS, Khor SP, Diasio R, Vonhoff DD, Burris HA (1998) Phase I clinical and pharmacologic study of eniluracil plus fluorouracil in patients with advanced cancer. J Clin Oncol 16:1450–1457
Porter DJT, Chestnut WG, Merrill BM, Spector T (1992) Mechanism-based inactivation of dihydropyrimidine dehydrogenase by 5-ethynyluracil. J Biol Chem 267:5236–5242
Miwa M, Ura M, Nishida M, Sawada N, Ishikawa T, Mori K, Shimma N, Umeda I, Ishitsuka H (1998) Design of a novel oral fluoropyrimidine carbamate, capecitabine, which generates 5-fluorouracil selectively in tumours by enzymes concentrated in human liver and cancer tissue. Eur J Cancer 34:1274–1281
Ackland SP, Peters GJ (1999) Thymidine phosphorylase: its role in sensitivity and resistance to anticancer drugs. Drug Res Updates 2:205–214
Peters GJ, Van Groeningen CJ, Laurensse EJ, Pinedo HM (1991) A comparison of 5-fluorouracil metabolism in human colorectal cancer and colon mucosa. Cancer 68:1903–1909
Humerickhouse RA, Dolan ME, Haraf DJ, Brockstein B, Stenson K, Kies M, Sulzen L, Ratain MJ, Vokes EE (1999) Phase I study of eniluracil, a dihydropyrimidine dehydrogenase inactivator, and oral 5-fluorouracil with radiation therapy in patients with recurrent or advanced head and neck cancer. Clin Cancer Res 5:291–298
Reigner B, Verweij J, Dirix L, Cassidy J, Twelves C, Allman D, Weidekamm E, Roos B, Banken L, Utoh M, Osterwalder B (1998) Effect of food on the pharmacokinetics of capecitabine and its metabolites following oral administration in cancer patients. Clin Cancer Res 4:941–948
Hirata K, Horikoshi N, Okazaki M, Denno R, Sasaki, K, Nakano Y, Ishizuka H, Yamada Y, Uno S, Taguchi T, Shirasaka T (1999) Pharmacokinetic study of S-1, a novel oral fluorouracil anti-tumor drug. Clin Cancer Res 5:2000–2005
Van Groeningen CJ, Peters GJ, Schornagel JH, Gall H, Noordhuis P, De Vries M, Turner SL, Swart MS, Pinedo HM, Hanauske AR, Giaccone G (2000) Phase I clinical and pharmacokinetic study of oral S-1 in patients with advanced solid tumors. J Clin Oncol 18:2772–2779
Borner MM, Schoffski P, De Wit R, Caponigro F, Comella G, Sulkes A, Greim G, Peters GJ, Van Der Born K, Wanders J, De Boer RF, Martin C, Fumoleau P (2002) Patient preference and pharmacokinetics of oral modulated UFT versus intravenous fluorouracil and leucovorin: a randomised crossover trial in advanced colorectal cancer. Eur J Cancer 38:349–358
Ho DH, Pazdur R, Covington W, Brown N, Huo YY, Lassere Y, Kuritani J (1998) Comparison of 5-fluorouracil pharmacokinetics in patients receiving continuous 5-fluorouracil infusion and oral uracil plus N1-(2′-tetrahydrofuranyl)-5-fluorouracil. Clin Cancer Res 4:2085–2088
Au JLS, Sadee W (1979) 5-Fluorouracil concentrations in human plasma following R,S-1-(tetrahydro-2-furanyl)-5-fluorouracil (Ftorafur) administration. Cancer Res 39:4289–4290
Manzuik LV, Perevodchikova NI, Gorbunova VA, Singin AS, Gerasimova GS, Bychkov MB, Rustum YM, Creavan PJ (1993) Initial clinical experience with oral ftorafur and 6R,S leucovorin in advanced colorectal carcinoma. Eur J Cancer 29A:1793–1794
Komatsu T, Yamazaki H, Shimada N, Nagayama S, Kawaguchi Y, Nakajima M, Yokoi T (2001) Involvement of microsomal cytochrome P450 and cytosolic thymidine phosphorylase in 5-fluorouracil formation from tegafur in human liver. Clin Cancer Res 7:675–681
Tatsumi K, Fukushima M, Shirasaka T, Fujii S (1987) Inhibitory effects of pyrimidine, barbituric acid and pyridine derivatives on 5-fluorouracil degradation in rat liver extracts. Jpn J Cancer Res 78:748–755
Naguib FN, El Kouhni MH, Cha S (1989) Structure-activity relationship of ligands of dihydrouracil dehydrogenase from mouse liver. Biochem Pharmacol 38:1471–1480
Fujita H, Okamoto M, Takao A, Matsushima E, Shirasaka T (1994) Preclinical studies of S-1, a new oral tegafur, plus modulators: pharmacokinetics. In: Einhorn J, Norrby R, Noorby SR, Nord CE (eds) Recent advances in chemotherapy: Proceedings of the 18th International Congress of Chemotherapy, Stockholm, Sweden, 27 June to 2 July 1993. ASM Press, Washington DC, pp 928–929
Takechi T, Nakano K, Uchida J, Mita A, Toko K, Takeda S, Unemi N, Shirasaka T (1997) Antitumor activity and low intestinal toxicity of S-1, a new formulation of oral tegafur, in experimental tumor models in rats. Cancer Chemother Pharmacol 39:205–211
Shirasaka T, Shimamoto Y, Fukushima M (1993) Inhibition by oxonic acid of gastrointestinal toxicity of 5-fluorouracil without loss of its antitumor activity in rats. Cancer Res 53:4004–4009
Matsushima E, Yoshida K, Kitamura R, Yoshida K (1997) Determination of S-1 (combined drug of tegafur, 5-chloro-2,4-dihydroxypyridine and potassium oxonate) and 5-fluorouracil in human plasma and urine using high-performance liquid chromatography and gas chromatography-negative ion chemical ionization mass spectrometry. J Chromatogr B Biomed Sci Appl 691:95–104
Van Kuilenburg AB, Stroomer AE, Peters GJ, Van Gennip AH (2001) Simultaneous determination of F-beta-alanine and beta-alanine in plasma and urine with dual-column reversed-phase high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl 759:51–61
Peters GJ, Lankelma J, Kok RM, Noordhuis P, Van Groeningen CJ, Van Der Wilt CL, Meyer S, Pinedo HM (1993) Prolonged retention of high concentrations of 5-fluorouracil in human and murine tumors as compared with plasma. Cancer Chemother Pharmacol 31:269–276
Van Kuilenburg ABP, Van Lenthe H, Tromp A, Veltman PCJ, Van Gennip AH (2000) Pitfalls in the diagnosis of patients with a partial dihydropyrimidine dehydrogenase deficiency. Clin Chem 46:9–17
Peters GJ, Van Der Wilt CL, Van Groeningen CJ, Smid K, Meijer S, Pinedo HM (1994) Thymidylate synthase inhibition after administration of fluorouracil with or without leucovorin in colon cancer patients: implications for treatment with fluorouracil. J Clin Oncol 12:2035–2042
Ahmed FY, Johnston SJ, Cassidy J, O'Kelly T, Binnie N, Murray GI, Van Gennip AH, Abeling NG, Knight S, McLeod HL (1999) Eniluracil treatment completely inactivates dihydropyrimidine dehydrogenase in colorectal tumors. J Clin Oncol 17:2439–2445
Shimada T, Yamazaki H, Guengerich P (1996) Ethnic-related differences in coumarin 7-hydroxylation activities catalyzed by cytochrome P4502A6 in liver microsomes of Japanese and Caucasian populations. Xenobiotica 26:395–405
Van Groeningen CJ, Pinedo HM, Heddes J, Kok RM, De Jong APJM, Wattel E, Peters GJ, Lankelma J (1988) Pharmacokinetics of 5-fluorouracil assessed with a sensitive mass spectrometric method in patients during a dose escalation schedule. Cancer Res 48:6956–6961
Peters GJ, Van Groeningen CJ, Giaccone G (2001) Fluorouracil (5-FU) pharmacokinetics in 5-FU prodrug formulations with a dihydropyrimidine dehydrogenase inhibitor. J Clin Oncol 19:4267–4268
Peters GJ, Backus HHJ, Freemantle S, Van Triest B, Codacci-Pisanelli G, Van Der Wilt CL, Smid K, Lunec J, Calvert AH, Marsh S, McLeod HL, Bloemena E, Meijer S, Jansen G, Van Groeningen CJ, Pinedo HM (2002) Induction of thymidylate synthase as a 5-fluorouracil resistance mechanism. Biochim Biophys Acta 1587:194–205
Abushullaih S, Saad ED, Munsell M, Hoff PM (2002) Incidence and severity of hand-foot syndrome in colorectal cancer patients treated with capecitabine: a single-institution experience. Cancer Invest 20:3–10
Poorter RL, Peters GJ, Bakker PJM, Taat CW, Biermans-Van Leeuwe DMJ, Codacci-Pisanelli G, Noordhuis P, Oosting J, Veenhof CHN (1995) Intermittent continuous infusion of 5-fluorouracil and low dose oral leucovorin in patients with gastrointestinal cancer: relationship between plasma concentrations and clinical parameters. Eur J Cancer 31A:1465–1470
Okeda R, Shibutani M, Matsuo T, Kuroiwa T, Shimokawa R, Tajima T (1990) Experimental neurotoxicity of 5-fluorouracil and its derivatives is due to poisoning by the monofluorinated organic metabolites, monofluoroacetic acid and α-fluoro-β-alanine. Acta Neuropathol 81:66–73
Koenig H, Patel A (1970) Biochemical basis for fluorouracil neurotoxicity. Arch Neurol 23:155–160
Peters GJ, Taguchi T, Hoff PM, Noordhuis P, Hirata K, Horikoshi N, Schornagel JH, Schellens JHM, Wanders J, Fumoleau P, Van der Born K, Van Groeningen CJ, Giaccone G (2002) Potential impact of interethnic differences on pharmacokinetics of S-1: relation with variations in toxicity. Proc Am Assoc Cancer Res 43:1012–1013 (no. 5017)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Peters, G.J., Noordhuis, P., Van Kuilenburg, A.B.P. et al. Pharmacokinetics of S-1, an oral formulation of ftorafur, oxonic acid and 5-chloro-2,4-dihydroxypyridine (molar ratio 1:0.4:1) in patients with solid tumors. Cancer Chemother Pharmacol 52, 1–12 (2003). https://doi.org/10.1007/s00280-003-0617-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-003-0617-9