Abstract
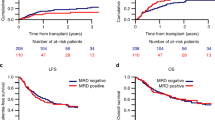
Measurable residual disease (MRD) monitoring independently predicts long-term outcomes in patients with acute myeloid leukemia (AML). Of the various modalities available, multiparameter flow cytometry–based MRD analysis is widely used and relevant for patients without molecular targets. In the transplant (HCT) setting, the presence of MRD pre-HCT is associated with adverse outcomes. MRD-negative remission status pre-HCT was also associated with longer overall (OS) and progression-free survival and a lower risk of relapse. We hypothesize that the combination of disease risk and MRD at the time of first complete remission (CR1) could identify patients according to the benefit gained from HCT, especially for intermediate-risk patients. We performed a retrospective analysis comparing the outcomes of HCT versus non-HCT therapies based on MRD status in AML patients who achieved CR1. Time-dependent analysis was applied considering time-to-HCT as a time-dependent covariate and compared HCT versus non-HCT outcomes according to MRD status at CR1. Among 336 patients assessed at CR1, 35.1% were MRD positive (MRDpos) post-induction. MRDpos patients benefitted from HCT with improved OS and relapse-free survival (RFS), while no benefit was observed in MRDneg patients. In adverse-risk patients, HCT improved OS (HR for OS 0.55; p = 0.05). In intermediate-risk patients, HCT benefit was not significant for OS and RFS. Intermediate-risk MRDpos patients were found to have benefit from HCT with improved OS (HR 0.45, p = 0.04), RFS (HR 0.46, p = 0.02), and CIR (HR 0.41, p = 0.02). Our data underscore the benefit of HCT in adverse risk and MRDpos intermediate-risk AML patients.



Similar content being viewed by others
Data availability
The datasets generated during and analyzed during the current study are available from the corresponding author on reasonable request.
References
Stein EM, Tallman MS (2012) Remission induction in acute myeloid leukemia. Int J Hematol 96:164–170. https://doi.org/10.1007/s12185-012-1121-y
Kantarjian H, Kadia T, DiNardo C, Daver N, Borthakur G, Jabbour E et al (2021) Acute myeloid leukemia: current progress and future directions. Blood Cancer J 11:41. https://doi.org/10.1038/s41408-021-00425-3
Döhner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Büchner T et al (2017) Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 129:424–447. https://doi.org/10.1182/blood-2016-08-733196
Cornelissen JJ, Blaise D (2016) Hematopoietic stem cell transplantation for patients with AML in first complete remission. Blood 127:62–70. https://doi.org/10.1182/blood-2015-07-604546
Cornelissen JJ, Gratwohl A, Schlenk RF, Sierra J, Bornhäuser M, Juliusson G et al (2012) The European LeukemiaNet AML Working Party consensus statement on allogeneic HSCT for patients with AML in remission: an integrated-risk adapted approach. Nat Rev Clin Oncol 9:579–590. https://doi.org/10.1038/nrclinonc.2012.150
Bornhäuser M, Schliemann C, Schetelig J, Röllig C, Kramer M, Glass B et al (2023) Allogeneic hematopoietic cell transplantation vs standard consolidation chemotherapy in patients with intermediate-risk acute myeloid leukemia: a randomized clinical trial. JAMA Oncol 9:519. https://doi.org/10.1001/jamaoncol.2022.7605
Schuurhuis GJ, Heuser M, Freeman S, Béné M-C, Buccisano F, Cloos J et al (2018) Minimal/measurable residual disease in AML: a consensus document from the European LeukemiaNet MRD Working Party. Blood 131:1275–1291. https://doi.org/10.1182/blood-2017-09-801498
Heuser M, Freeman SD, Ossenkoppele GJ, Buccisano F, Hourigan CS, Ngai LL et al (2021) 2021 Update on MRD in acute myeloid leukemia: a consensus document from the European LeukemiaNet MRD Working Party. Blood 138:2753–2767. https://doi.org/10.1182/blood.2021013626
Luger SM (2021) Consolidation therapy for acute myeloid leukemia: defining a benchmark. J Clin Oncol 39:870–875. https://doi.org/10.1200/JCO.20.03142
Freeman SD, Virgo P, Couzens S, Grimwade D, Russell N, Hills RK et al (2013) Prognostic relevance of treatment response measured by flow cytometric residual disease detection in older patients with acute myeloid leukemia. J Clin Oncol 31:4123–4131. https://doi.org/10.1200/JCO.2013.49.1753
Balsat M, Renneville A, Thomas X, de Botton S, Caillot D, Marceau A et al (2017) Postinduction minimal residual disease predicts outcome and benefit from allogeneic stem cell transplantation in acute myeloid leukemia with NPM1 mutation: a study by the acute leukemia French Association Group. J Clin Oncol 35:185–193. https://doi.org/10.1200/JCO.2016.67.1875
Freeman SD, Hills RK, Virgo P, Khan N, Couzens S, Dillon R et al (2018) Measurable residual disease at induction redefines partial response in acute myeloid leukemia and stratifies outcomes in patients at standard risk without NPM1 mutations. J Clin Oncol 36:1486–1497. https://doi.org/10.1200/JCO.2017.76.3425
Araki D, Wood BL, Othus M, Radich JP, Halpern AB, Zhou Y et al (2016) Allogeneic hematopoietic cell transplantation for acute myeloid leukemia: time to move toward a minimal residual disease–based definition of complete remission? J Clin Oncol 34:329–336. https://doi.org/10.1200/JCO.2015.63.3826
Yu S, Fan Z, Ma L, Wang Y, Huang F, Zhang Q et al (2021) Association between measurable residual disease in patients with intermediate-risk acute myeloid leukemia and first remission, treatment, and outcomes. JAMA Netw Open 4:e2115991. https://doi.org/10.1001/jamanetworkopen.2021.15991
Venditti A, Piciocchi A, Candoni A, Melillo L, Calafiore V, Cairoli R et al (2019) GIMEMA AML1310 trial of risk-adapted, MRD-directed therapy for young adults with newly diagnosed acute myeloid leukemia. Blood 134:935–945. https://doi.org/10.1182/blood.2018886960
Venditti A, Piciocchi A, Palmieri R, Arena V, Candoni A, Calafiore V et al (2021) Results of the 6-year follow-up of the gimema AML1310 trial: a risk-adapted, MRD-directed therapy for young adults with newly diagnosed acute myeloid leukemia. Blood 138:2359–2359. https://doi.org/10.1182/blood-2021-147200
Delgado J, Pereira A, Villamor N, Lopez-Guillermo A, Rozman C (2014) Survival analysis in hematologic malignancies: recommendations for clinicians. Haematologica 99:1410–1420. https://doi.org/10.3324/haematol.2013.100784
Schultz LR, Peterson EL, Breslau N (2002) Graphing survival curve estimates for time-dependent covariates. Int J Methods Psychiatr Res 11:68–74. https://doi.org/10.1002/mpr.124
Kanda Y (2013) Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 48:452–458. https://doi.org/10.1038/bmt.2012.244
Grimwade D, Hills RK, Moorman AV, Walker H, Chatters S, Goldstone AH et al (2010) Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood 116:354–365. https://doi.org/10.1182/blood-2009-11-254441
Veltri L, Rezvani K, Oran B, Mehta R, Rondon G, Kebriaei P et al (2019) Allotransplants for patients 65 years or older with high-risk acute myeloid leukemia. Biol Blood Marrow Transplant 25:505–514. https://doi.org/10.1016/j.bbmt.2018.09.032
Walter RB, Gyurkocza B, Storer BE, Godwin CD, Pagel JM, Buckley SA et al (2015) Comparison of minimal residual disease as outcome predictor for AML patients in first complete remission undergoing myeloablative or nonmyeloablative allogeneic hematopoietic cell transplantation. Leukemia 29:137–144. https://doi.org/10.1038/leu.2014.173
Hourigan CS, Dillon LW, Gui G, Logan BR, Fei M, Ghannam J et al (2020) Impact of conditioning intensity of allogeneic transplantation for acute myeloid leukemia with genomic evidence of residual disease. J Clin Oncol 38:1273–1283. https://doi.org/10.1200/JCO.19.03011
Ahn J-S, Kim T, Jung S-H, Ahn S-Y, Jung S-Y, Song G-Y et al (2021) Allogeneic transplant can abrogate the risk of relapse in the patients of first remission acute myeloid leukemia with detectable measurable residual disease by next-generation sequencing. Bone Marrow Transplant 56:1159–1170. https://doi.org/10.1038/s41409-020-01165-x
Dillon LW, Gui G, Page KM, Ravindra N, Wong ZC, Andrew G et al (2023) DNA sequencing to detect residual disease in adults with acute myeloid leukemia prior to hematopoietic cell transplant. JAMA 329:745. https://doi.org/10.1001/jama.2023.1363
Grob T, Sanders MA, Vonk CM, Kavelaars FG, Rijken M, Hanekamp DW et al (2023) Prognostic value of FLT3 -internal tandem duplication residual disease in acute myeloid leukemia. J Clin Oncol 41:756–765. https://doi.org/10.1200/JCO.22.00715
Loo S, Dillon R, Ivey A, Anstee NS, Othman J, Tiong IS et al (2022) Pretransplant FLT3 -ITD MRD assessed by high-sensitivity PCR-NGS determines posttransplant clinical outcome. Blood 140:2407–2411. https://doi.org/10.1182/blood.2022016567
Fasan O (2019) Using minimal (measurable) residual disease assessments to guide decision-making for timing of allogeneic transplantation in acute myeloid leukemia. Curr Opin Hematol 26:413–20. https://doi.org/10.1097/MOH.0000000000000543
Platzbecker U, Middeke JM, Sockel K, Herbst R, Wolf D, Baldus CD et al (2018) Measurable residual disease-guided treatment with azacitidine to prevent haematological relapse in patients with myelodysplastic syndrome and acute myeloid leukaemia (RELAZA2): an open-label, multicentre, phase 2 trial. Lancet Oncol 19:1668–1679. https://doi.org/10.1016/S1470-2045(18)30580-1
Author information
Authors and Affiliations
Contributions
JL and MA acquired data, interpreted results, drafted, and revised the manuscript. VG, ACS, and JM provided feedback on data analysis and the manuscript. INB revised the manuscript. JMC, TS, AB, SC, MM, RK, HS, and AT approved the final version. DDK acquired data, interpreted results, revised, and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent to participate
Informed consent was not obtained due to the retrospective nature of the review of data. All personal health information has been de-identified.
Conflict of interest
JL, MA, INB, JMC, TS, AB, SC, ACS, MM, JM, RK, HS, AT, and DDK declare that they have no conflict of interest. VG reports consulting fees from AbbVie, Bristol Myers Squibb/Celgene, Constellation Biopharma, Novartis, Pfizer, and Sierra Oncology; honoraria from Bristol Myers Squibb/Celgene, Constellation Biopharma, and Novartis; and participation in the data safety monitoring board or advisory board for AbbVie, Bristol Myers Squibb/Celgene, Pfizer, and Roche.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
On behalf of the PMH MRD working group.
JL and MA contributed to the work equally as co-first authors.
HS, AT, and DDK contributed to the work equally as co-senior authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lucero, J., Alhumaid, M., Novitzky-Basso, I. et al. Flow cytometry-based measurable residual disease (MRD) analysis identifies AML patients who may benefit from allogeneic hematopoietic stem cell transplantation. Ann Hematol 103, 1187–1196 (2024). https://doi.org/10.1007/s00277-024-05639-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-024-05639-6