Abstract
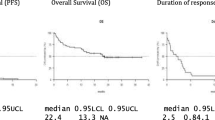
The anti-CD38 monoclonal antibody daratumumab is approved as a single agent for the treatment of patients with relapsed and refractory multiple myeloma (RRMM) who have received at least three prior lines of therapy, including a proteasome inhibitor (PI) and an immunomodulatory agent (IMID), or who are double refractory to a PI and an IMID. To date, no real-life data on the efficacy and tolerance of daratumumab in this setting are available. We report here the results of a single-center series of 41 RRMM patients treated with single-agent daratumumab outside clinical trials. Patients received a median number of 4 prior therapies. All patients were previously exposed to PI and IMID and all patients were refractory to the last line of therapy. Most patients presented with high-risk characteristics, including 24% adverse cytogenetics (del17p/t(4,14)), 31% extramedullary disease and 12% circulating plasmacytosis at time of daratumumab therapy. The overall response rate was 24%, including 5% very good partial response or better. After a median follow-up of 6.5 months, all patients experienced disease relapse. The median progression-free survival was 1.9 months. At the time of disease progression, 44% of patients did not receive subsequent therapy. The median overall survival was 6.5 months. No new safety signal was identified. These real-life results revealed modest efficacy of single-agent daratumumab in advanced patients with RRMM in comparison with data from clinical trials.


Similar content being viewed by others
References
Kumar SK, Rajkumar SV, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, Zeldenrust SR, Dingli D, Russell SJ, Lust JA, Greipp PR, Kyle RA, Gertz MA (2008) Improved survival in multiple myeloma and the impact of novel therapies. Blood 111:2516–2520. https://doi.org/10.1182/blood-2007-10-116129
Kumar SK, Dimopoulos MA, Kastritis E, Terpos E, Nahi H, Goldschmidt H, Hillengass J, Leleu X, Beksac M, Alsina M, Oriol A, Cavo M, Ocio EM, Mateos MV, O'Donnell EK, Vij R, Lokhorst HM, van de Donk NWCJ, Min C, Mark T, Turesson I, Hansson M, Ludwig H, Jagannath S, Delforge M, Kyriakou C, Hari P, Mellqvist U, Usmani SZ, Dytfeld D, Badros AZ, Moreau P, Kim K, Otero PR, Lee JH, Shustik C, Waller D, Chng WJ, Ozaki S, Lee JJ, de la Rubia J, Eom HS, Rosinol L, Lahuerta JJ, Sureda A, Kim JS, Durie BGM (2017) Natural history of relapsed myeloma, refractory to immunomodulatory drugs and proteasome inhibitors: a multicenter IMWG study. Leukemia 31:2443–2448. https://doi.org/10.1038/leu.2017.138
Touzeau C, Moreau P, Dumontet C (2017) Monoclonal antibody therapy in multiple myeloma. Leukemia 31:1039–1047. https://doi.org/10.1038/leu.2017.60
Lokhorst HM, Plesner T, Laubach JP, Nahi H, Gimsing P, Hansson M, Minnema MC, Lassen U, Krejcik J, Palumbo A, van de Donk NWCJ, Ahmadi T, Khan I, Uhlar CM, Wang J, Sasser AK, Losic N, Lisby S, Basse L, Brun N, Richardson PG (2015) Targeting CD38 with daratumumab monotherapy in multiple myeloma. N Engl J Med 373:1207–1219. https://doi.org/10.1056/NEJMoa1506348
de Weers M, Tai Y-T, van der Veer MS et al (2011) Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J Immunol Baltim Md 1950 186:1840–1848. https://doi.org/10.4049/jimmunol.1003032
Lonial S, Weiss BM, Usmani SZ, Singhal S, Chari A, Bahlis NJ, Belch A, Krishnan A, Vescio RA, Mateos MV, Mazumder A, Orlowski RZ, Sutherland HJ, Bladé J, Scott EC, Oriol A, Berdeja J, Gharibo M, Stevens DA, LeBlanc R, Sebag M, Callander N, Jakubowiak A, White D, de la Rubia J, Richardson PG, Lisby S, Feng H, Uhlar CM, Khan I, Ahmadi T, Voorhees PM (2016) Daratumumab monotherapy in patients with treatment-refractory multiple myeloma (SIRIUS): an open-label, randomised, phase 2 trial. Lancet Lond Engl 387:1551–1560. https://doi.org/10.1016/S0140-6736(15)01120-4
Usmani SZ, Weiss BM, Plesner T, Bahlis NJ, Belch A, Lonial S, Lokhorst HM, Voorhees PM, Richardson PG, Chari A, Sasser AK, Axel A, Feng H, Uhlar CM, Wang J, Khan I, Ahmadi T, Nahi H (2016) Clinical efficacy of daratumumab monotherapy in patients with heavily pretreated relapsed or refractory multiple myeloma. Blood 128:37–44. https://doi.org/10.1182/blood-2016-03-705210
Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos MV, Kumar S, Hillengass J, Kastritis E, Richardson P, Landgren O, Paiva B, Dispenzieri A, Weiss B, LeLeu X, Zweegman S, Lonial S, Rosinol L, Zamagni E, Jagannath S, Sezer O, Kristinsson SY, Caers J, Usmani SZ, Lahuerta JJ, Johnsen HE, Beksac M, Cavo M, Goldschmidt H, Terpos E, Kyle RA, Anderson KC, Durie BGM, Miguel JFS (2014) International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol 15:e538–e548. https://doi.org/10.1016/S1470-2045(14)70442-5
Kumar S, Paiva B, Anderson KC, Durie B, Landgren O, Moreau P, Munshi N, Lonial S, Bladé J, Mateos MV, Dimopoulos M, Kastritis E, Boccadoro M, Orlowski R, Goldschmidt H, Spencer A, Hou J, Chng WJ, Usmani SZ, Zamagni E, Shimizu K, Jagannath S, Johnsen HE, Terpos E, Reiman A, Kyle RA, Sonneveld P, Richardson PG, McCarthy P, Ludwig H, Chen W, Cavo M, Harousseau JL, Lentzsch S, Hillengass J, Palumbo A, Orfao A, Rajkumar SV, Miguel JS, Avet-Loiseau H (2016) International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol 17:e328–e346. https://doi.org/10.1016/S1470-2045(16)30206-6
Chari A, Lonial S, Mark TM, Krishnan AY, Stockerl-Goldstein KE, Usmani SZ, Londhe A, Etheredge D, Fleming S, Liu B, Ukropec J, Lin TS, Jagannath S, Nooka AK (2018) Results of an early access treatment protocol of daratumumab in United States patients with relapsed or refractory multiple myeloma. Cancer 124:4342–4349. https://doi.org/10.1002/cncr.31706
Dimopoulos MA, Oriol A, Nahi H, San-Miguel J, Bahlis NJ, Usmani SZ, Rabin N, Orlowski RZ, Komarnicki M, Suzuki K, Plesner T, Yoon SS, Ben Yehuda D, Richardson PG, Goldschmidt H, Reece D, Lisby S, Khokhar NZ, O'Rourke L, Chiu C, Qin X, Guckert M, Ahmadi T, Moreau P, POLLUX Investigators (2016) Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med 375:1319–1331. https://doi.org/10.1056/NEJMoa1607751
Palumbo A, Chanan-Khan A, Weisel K, Nooka AK, Masszi T, Beksac M, Spicka I, Hungria V, Munder M, Mateos MV, Mark TM, Qi M, Schecter J, Amin H, Qin X, Deraedt W, Ahmadi T, Spencer A, Sonneveld P, CASTOR Investigators (2016) Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med 375:754–766. https://doi.org/10.1056/NEJMoa1606038
Mateos M-V, Dimopoulos MA, Cavo M, Suzuki K, Jakubowiak A, Knop S, Doyen C, Lucio P, Nagy Z, Kaplan P, Pour L, Cook M, Grosicki S, Crepaldi A, Liberati AM, Campbell P, Shelekhova T, Yoon SS, Iosava G, Fujisaki T, Garg M, Chiu C, Wang J, Carson R, Crist W, Deraedt W, Nguyen H, Qi M, San-Miguel J, ALCYONE Trial Investigators (2017) Daratumumab plus bortezomib, melphalan, and prednisone for untreated myeloma. N Engl J Med 378:518–528. https://doi.org/10.1056/NEJMoa1714678
Facon T, Kumar SK, Plesner T et al (2018) Phase 3 randomized study of daratumumab plus lenalidomide and dexamethasone (D-Rd) versus lenalidomide and dexamethasone (Rd) in patients with newly diagnosed multiple myeloma (NDMM) ineligible for transplant (MAIA). Blood 132:LBA-2-LBA-2. https://doi.org/10.1182/blood-2018-120737
Acknowledgments
CT, PM, and SLG are members of Site de Recherche Intégrée sur le Cancer (SIRIC) “ILIAD,” (INCA-DGOS-Inserm_12558).
Author information
Authors and Affiliations
Contributions
CT PM and MJ designed the study. MJ collected data. MJ performed statistical analysis. CT and MJ wrote the manuscript. All authors treated patients and critically reviewed the manuscript and gave final approval.
Corresponding author
Ethics declarations
Conflict of interest
CT and PM are advisory board member and received honoraria from Janssen. Other authors have no conflict of interest to declare.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jullien, M., Trudel, S., Tessoulin, B. et al. Single-agent daratumumab in very advanced relapsed and refractory multiple myeloma patients: a real-life single-center retrospective study. Ann Hematol 98, 1435–1440 (2019). https://doi.org/10.1007/s00277-019-03655-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-019-03655-5