Abstract
CD19 chimeric antigen receptor (CAR) T cell therapy has shown impressive results in treating acute lymphoblastic leukemia (B-ALL), chronic lymphoblastic leukemia (B-CLL), and B-cell non-Hodgkin lymphoma (B-NHL) over the past few years. Meanwhile, the cytokine release syndrome (CRS), which could be moderate or even life-threatening, has emerged as the most significant adverse effect in the clinical course of this novel targeting immunotherapy. In this systematic review, we analyzed the incidence of severe CRS in 19 clinical trials selected from studies published between 2010 and 2017. The pooled severe CRS proportion was 29.3% (95% confidence interval [CI] 12.3–49.1%) in B-ALL, 38.8% (95%CI 12.9–67.6%) in B-CLL, and 19.8% (95%CI 4.2–40.8%) in B-NHL. In the univariate meta regression analysis, the proliferation of CD19-CAR-T cell in vivo was correlated with the severe CRS. Specifically, total infusion cell dose contributed to the severe CRS occurring in B-ALL patients but not in B-CLL or B-NHL patients. Tumor burden was strongly associated with the severity of CRS in B-ALL. Besides, post-HSCT CD19 CAR-T cell infusion represented lower severe CRS incidence. Further investigations into the risk factors of CRS in B-CLL and B-NHL are needed.



Similar content being viewed by others
References
Brudno JN, Kochenderfer JN (2016) Toxicities of chimeric antigen receptor T cells: recognition and management. Blood 127(26):3321–3330. https://doi.org/10.1182/blood-2016-04-703751
Kochenderfer JN, Dudley ME, Feldman SA, Wilson WH, Spaner DE, Maric I, Stetler-Stevenson M, Phan GQ, Hughes MS, Sherry RM, Yang JC, Kammula US, Devillier L, Carpenter R, Nathan DA, Morgan RA, Laurencot C, Rosenberg SA (2012) B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood 119(12):2709–2720. https://doi.org/10.1182/blood-2011-10-384388
Brentjens RJ, Riviere I, Park JH, Davila ML, Wang X, Stefanski J, Taylor C, Yeh R, Bartido S, Borquez-Ojeda O, Olszewska M, Bernal Y, Pegram H, Przybylowski M, Hollyman D, Usachenko Y, Pirraglia D, Hosey J, Santos E, Halton E, Maslak P, Scheinberg D, Jurcic J, Heaney M, Heller G, Frattini M, Sadelain M (2011) Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood 118(18):4817–4828. https://doi.org/10.1182/blood-2011-04-348540
Locke FL, Neelapu SS, Bartlett NL, Siddiqi T, Chavez JC, Hosing CM, Ghobadi A, Budde LE, Bot A, Rossi JM, Jiang Y, Xue AX, Elias M, Aycock J, Wiezorek J, Go WY (2017) Phase 1 results of ZUMA-1: a multicenter study of KTE-C19 anti-CD19 CAR T cell therapy in refractory aggressive lymphoma. Mol Ther : J Am Soc Gene Ther 25(1):285–295. https://doi.org/10.1016/j.ymthe.2016.10.020
Cruz CR, Micklethwaite KP, Savoldo B, Ramos CA, Lam S, Ku S, Diouf O, Liu E, Barrett AJ, Ito S, Shpall EJ, Krance RA, Kamble RT, Carrum G, Hosing CM, Gee AP, Mei Z, Grilley BJ, Heslop HE, Rooney CM, Brenner MK, Bollard CM, Dotti G (2013) Infusion of donor-derived CD19-redirected virus-specific T cells for B-cell malignancies relapsed after allogeneic stem cell transplant: a phase 1 study. Blood 122(17):2965–2973. https://doi.org/10.1182/blood-2013-06-506741
Dai H, Zhang W, Li X, Han Q, Guo Y, Zhang Y, Wang Y, Wang C, Shi F, Zhang Y, Chen M, Feng K, Wang Q, Zhu H, Fu X, Li S, Han W (2015) Tolerance and efficacy of autologous or donor-derived T cells expressing CD19 chimeric antigen receptors in adult B-ALL with extramedullary leukemia. Oncoimmunology 4(11):e1027469. https://doi.org/10.1080/2162402X.2015.1027469
Brudno JN, Somerville RP, Shi V, Rose JJ, Halverson DC, Fowler DH, Gea-Banacloche JC, Pavletic SZ, Hickstein DD, Lu TL, Feldman SA, Iwamoto AT, Kurlander R, Maric I, Goy A, Hansen BG, Wilder JS, Blacklock-Schuver B, Hakim FT, Rosenberg SA, Gress RE, Kochenderfer JN (2016) Allogeneic T cells that express an anti-CD19 chimeric antigen receptor induce remissions of B-cell malignancies that progress after allogeneic hematopoietic stem-cell transplantation without causing graft-versus-host disease. J Clin Oncol : Off J Am Soc Clin Oncol 34(10):1112–1121. https://doi.org/10.1200/JCO.2015.64.5929
Wang X, Popplewell LL, Wagner JR, Naranjo A, Blanchard MS, Mott MR, Norris AP, Wong CW, Urak RZ, Chang WC, Khaled SK, Siddiqi T, Budde LE, Xu J, Chang B, Gidwaney N, Thomas SH, Cooper LJ, Riddell SR, Brown CE, Jensen MC, Forman SJ (2016) Phase 1 studies of central memory-derived CD19 CAR T-cell therapy following autologous HSCT in patients with B-cell NHL. Blood 127(24):2980–2990. https://doi.org/10.1182/blood-2015-12-686725
Kebriaei P, Singh H, Huls MH, Figliola MJ, Bassett R, Olivares S, Jena B, Dawson MJ, Kumaresan PR, Su S, Maiti S, Dai J, Moriarity B, Forget MA, Senyukov V, Orozco A, Liu T, McCarty J, Jackson RN, Moyes JS, Rondon G, Qazilbash M, Ciurea S, Alousi A, Nieto Y, Rezvani K, Marin D, Popat U, Hosing C, Shpall EJ, Kantarjian H, Keating M, Wierda W, Do KA, Largaespada DA, Lee DA, Hackett PB, Champlin RE, Cooper LJ (2016) Phase I trials using sleeping beauty to generate CD19-specific CAR T cells. J Clin Invest 126(9):3363–3376. https://doi.org/10.1172/jci86721
Savoldo B, Ramos CA, Liu E, Mims MP, Keating MJ, Carrum G, Kamble RT, Bollard CM, Gee AP, Mei Z, Liu H, Grilley B, Rooney CM, Heslop HE, Brenner MK, Dotti G (2011) CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J Clin Invest 121(5):1822–1826. https://doi.org/10.1172/jci46110
Kochenderfer JN, Dudley ME, Carpenter RO, Kassim SH, Rose JJ, Telford WG, Hakim FT, Halverson DC, Fowler DH, Hardy NM, Mato AR, Hickstein DD, Gea-Banacloche JC, Pavletic SZ, Sportes C, Maric I, Feldman SA, Hansen BG, Wilder JS, Blacklock-Schuver B, Jena B, Bishop MR, Gress RE, Rosenberg SA (2013) Donor-derived CD19-targeted T cells cause regression of malignancy persisting after allogeneic hematopoietic stem cell transplantation. Blood 122(25):4129–4139. https://doi.org/10.1182/blood-2013-08-519413
Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K, Chung SS, Stefanski J, Borquez-Ojeda O, Olszewska M, Qu J, Wasielewska T, He Q, Fink M, Shinglot H, Youssif M, Satter M, Wang Y, Hosey J, Quintanilla H, Halton E, Bernal Y, Bouhassira DC, Arcila ME, Gonen M, Roboz GJ, Maslak P, Douer D, Frattini MG, Giralt S, Sadelain M, Brentjens R (2014) Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med 6(224):224ra225. https://doi.org/10.1126/scitranslmed.3008226
Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF, Mahnke YD, Melenhorst JJ, Rheingold SR, Shen A, Teachey DT, Levine BL, June CH, Porter DL, Grupp SA (2014) Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 371(16):1507–1517. https://doi.org/10.1056/NEJMoa1407222
Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, Fry TJ, Orentas R, Sabatino M, Shah NN, Steinberg SM, Stroncek D, Tschernia N, Yuan C, Zhang H, Zhang L, Rosenberg SA, Wayne AS, Mackall CL (2015) T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet 385(9967):517–528. https://doi.org/10.1016/S0140-6736(14)61403-3
Kochenderfer JN, Dudley ME, Kassim SH, Somerville RP, Carpenter RO, Stetler-Stevenson M, Yang JC, Phan GQ, Hughes MS, Sherry RM, Raffeld M, Feldman S, Lu L, Li YF, Ngo LT, Goy A, Feldman T, Spaner DE, Wang ML, Chen CC, Kranick SM, Nath A, Nathan DA, Morton KE, Toomey MA, Rosenberg SA (2015) Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol : Off J Am Soc Clin Oncol 33(6):540–549. https://doi.org/10.1200/JCO.2014.56.2025
Porter DL, Hwang WT, Frey NV, Lacey SF, Shaw PA, Loren AW, Bagg A, Marcucci KT, Shen A, Gonzalez V, Ambrose D, Grupp SA, Chew A, Zheng Z, Milone MC, Levine BL, Melenhorst JJ, June CH (2015) Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med 7(303):303ra139. https://doi.org/10.1126/scitranslmed.aac5415
Turtle CJ, Hanafi LA, Berger C, Gooley TA, Cherian S, Hudecek M, Sommermeyer D, Melville K, Pender B, Budiarto TM, Robinson E, Steevens NN, Chaney C, Soma L, Chen X, Yeung C, Wood B, Li D, Cao J, Heimfeld S, Jensen MC, Riddell SR, Maloney DG (2016) CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Invest 126(6):2123–2138. https://doi.org/10.1172/JCI85309
Turtle CJ, Hanafi LA, Berger C, Hudecek M, Pender B, Robinson E, Hawkins R, Chaney C, Cherian S, Chen X, Soma L, Wood B, Li D, Heimfeld S, Riddell SR, Maloney DG (2016) Immunotherapy of non-Hodgkin’s lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor-modified T cells. Sci Transl Med 8(355):355ra116. https://doi.org/10.1126/scitranslmed.aaf8621
Hu Y, Wu Z, Luo Y, Shi J, Yu J, Pu C, Liang Z, Wei G, Cui Q, Sun J, Jiang J, Xie J, Tan Y, Ni W, Tu J, Wang J, Jin A, Zhang H, Cai Z, Xiao L, Huang H (2016) Potent anti-leukemia activities of chimeric antigen receptor modified T cells against CD19 in Chinese patients with relapsed/refractory acute lymphocytic leukemia. Clin Cancer Res : Off J Am Assoc Cancer Res 23:3297–3306. https://doi.org/10.1158/1078-0432.ccr-16-1799
Kochenderfer JN, Somerville RPT, Lu T, Shi V, Bot A, Rossi J, Xue A, Goff SL, Yang JC, Sherry RM, Klebanoff CA, Kammula US, Sherman M, Perez A, Yuan CM, Feldman T, Friedberg JW, Roschewski MJ, Feldman SA, McIntyre L, Toomey MA, Rosenberg SA (2017) Lymphoma remissions caused by anti-CD19 chimeric antigen receptor T cells are associated with high serum interleukin-15 levels. J Clin Oncol : Off J Am Soc Clin Oncol 35(16):1803–1813. https://doi.org/10.1200/JCO.2016.71.3024
Frey NV, Porter DL (2016) Cytokine release syndrome with novel therapeutics for acute lymphoblastic leukemia. Hematol Ed Program Ame Soc Hematol Am Soc Hematol Ed Program 2016(1):567–572. https://doi.org/10.1182/asheducation-2016.1.567
Anwer F, Shaukat AA, Zahid U, Husnain M, McBride A, Persky D, Lim M, Hasan N, Riaz IB (2017) Donor origin CAR T cells: graft versus malignancy effect without GVHD, a systematic review. Immunotherapy 9(2):123–130. https://doi.org/10.2217/imt-2016-0127
Kawalekar OU, O'Connor RS, Fraietta JA, Guo L, McGettigan SE, Posey AD Jr, Patel PR, Guedan S, Scholler J, Keith B, Snyder N, Blair I, Milone MC, June CH (2016) Distinct signaling of coreceptors regulates specific metabolism pathways and impacts memory development in CAR T cells. Immunity 44(2):380–390. https://doi.org/10.1016/j.immuni.2016.01.021
Watanabe K, Terakura S, Martens AC, van Meerten T, Uchiyama S, Imai M, Sakemura R, Goto T, Hanajiri R, Imahashi N, Shimada K, Tomita A, Kiyoi H, Nishida T, Naoe T, Murata M (2015) Target antigen density governs the efficacy of anti-CD20-CD28-CD3 zeta chimeric antigen receptor-modified effector CD8+ T cells. J Immunol (Baltimore, Md : 1950) 194(3):911–920. https://doi.org/10.4049/jimmunol.1402346
Teachey DT, Lacey SF, Shaw PA, Melenhorst JJ, Maude SL, Frey N, Pequignot E, Gonzalez VE, Chen F, Finklestein J, Barrett DM, Weiss SL, Fitzgerald JC, Berg RA, Aplenc R, Callahan C, Rheingold SR, Zheng Z, Rose-John S, White JC, Nazimuddin F, Wertheim G, Levine BL, June CH, Porter DL, Grupp SA (2016) Identification of predictive biomarkers for cytokine release syndrome after chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Cancer Discov 6(6):664–679. https://doi.org/10.1158/2159-8290.CD-16-0040
Hay KA, Hanafi LA, Li D, Gust J, Liles WC, Wurfel MM, Lopez JA, Chen J, Chung D, Harju-Baker S, Cherian S, Chen X, Riddell SR, Maloney DG, Turtle CJ (2017) Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor-modified T-cell therapy. Blood 130(21):2295–2306. https://doi.org/10.1182/blood-2017-06-793141
David MB, Nathan S, Ted JH, Zachary G, Stephan AG (2016) Interleukin 6 is not made by chimeric antigen receptor T cells and does not impact their function. 58th Am Soc Hematol Annu Meet Exposition https://ash.confex.com/ash/2016/webprogram/Paper92806.html
Norelli M, Casucci M, Bonini C, Bondanza A (2016) Clinical pharmacology of CAR-T cells: linking cellular pharmacodynamics to pharmacokinetics and antitumor effects. Biochim Biophys Acta 1865(1):90–100. https://doi.org/10.1016/j.bbcan.2015.12.001
Funding
This study was supported by National Natural Sciences Foundation of China (81600151) and Shanghai Municipal Bureau of health and Family Planning Commission (Grants 20154Y0168).
Author information
Authors and Affiliations
Contributions
Zhen Jin designed the study, collected the data, preformed the statistical analysis, and wrote the manuscripts. Rufang Xiang collected all the data, performed the statistical analysis, and revised the manuscript. Kai Qing, Xiaoyang Li, Yunxiang Zhang, Hongming Zhu, Lining Wang, and Yuanfei Mao revised the paper. Junmin Li and Zizhen Xu designed the study and revised the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Figure S1
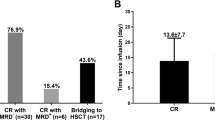
Forest plot of severe CRS incidence in post-HSCT CAR-T cell infusion group and non-HSCT patients group based on disease type. (GIF 97 kb)
Rights and permissions
About this article
Cite this article
Jin, Z., Xiang, R., Qing, K. et al. The severe cytokine release syndrome in phase I trials of CD19-CAR-T cell therapy: a systematic review. Ann Hematol 97, 1327–1335 (2018). https://doi.org/10.1007/s00277-018-3368-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-018-3368-8