Abstract
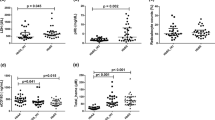
Sickle cell patients are characterized by stress erythropoiesis involving cytokines, growth factors, and adhesion molecules. We set out to determine whether serum soluble vascular cell adhesion molecule-1 (sVCAM-1) levels, which are inversely related to red blood cell counts in sickle cell disease (SCD), reflect erythropoietic activity in adult HbSS patients. Serum levels of sVCAM-1 were compared to erythropoietin (EPO), granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin-3 (IL-3), and soluble transferrin receptor (sTfR) levels in 29 adults with HbSS, and their respective levels were also compared to 29 race- and age-matched HbAA controls. EPO and sTfR levels were increased as compared to healthy controls, whereas IL-3 and GM-CSF were not. No significant correlation of sVCAM-1 levels could be detected with any of the measured erythropoietic markers. Patients, but not controls, with detectable IL-3 levels had lower sTfR and GM-CSF levels as compared to patients with undetectable IL-3 levels. Even though a link of sVCAM-1 to erythropoiesis could not be established, it cannot be ruled out that sVCAM-1 levels reflect the release of young red blood cells into the circulation. IL-3 and GM-CSF levels suggest that different rates of erythropoiesis may be characterized by specific cytokine profiles in SCD. Further research should focus on the potential cytokines and adhesion molecules involved in sickle cell erythropoiesis, as this may increase our understanding of sickle cell complications and may provide us with potential markers for risk assessment in sickle cell disease as well.

Similar content being viewed by others
References
Yi-Hsin Chan J, Watt SM (2000) Adhesion receptors on haematopoietic progenitor cells. Br J Haematol 112:541–557
Jacobsen K, Kravitz J, Kincade PW, Osmond DG (1996) Adhesion receptors on bone marrow stromal cells: in vivo expression of vascular cell adhesion molecule-1 by reticular cells and sinusoidal endothelium in normal and γ-irradiated mice. Blood 87:73–82
Serjeant GR (2001) Sickle cell disease. Oxford Press, New York
Duits AJ, Pieters RC, Saleh AW, van Rosmalen E, Katenberg H, Berend K, Rojer RA (1996) Enhanced levels of soluble VCAM-1 in sickle cell patients and their specific increment during vasoocclusive crisis. Clin Immunol Immunopathol 81:96–98
Schnog JB, De Vries C, Van der Dijs FPL, Muskiet FD, Muskiet FAJ, Duits AJ (1998) Enhanced sVCAM-1 levels in pediatric patients with sickle cell disease (abstract). West Indian Med J 47 [Suppl 2]:55a
Saleh AW, Hillen HFP, Duits AJ (1999) Levels of endothelial, neutrophil and platelet specific factors in sickle cell anemia patients during hydroxyurea treatment. Acta Haematol 102:31–37
Croizat H, Nagel R (1999) Circulating cytokines response and the levels of erythropoiesis in sickle cell anemia. Am J Hematol 60:105–115
Schnog JB, Keli SO, Pieters RA, Rojer RA, Duits AJ (2000) Duffy phenotype does not influence sickle cell disease severity. Clin Immunol 96:264–268
Van der Dijs FPL, Van den Berg GA, Schermer JG, Muskiet FD, Landman H, Muskiet FAJ (1992) Screening cord blood for hemoglobinopathies and thalassemia by HPLC. Clin Chem 38:1864–1869
Bondurant MC, Koury MJ (1999) Hematopoiesis. In: Lee GR, Lukens J, Greer JP, Rodgers GM, Paraskevas F, Foerster J (eds) Wintrobe's clinical hematology, 10th edn. Lippincott Williams & Wilkins, Philadelphia
Dosquet C, Chen Y, Makke J, Miclea JM, Coudert MC, Marolleau JP, Fermand JP, Cottu P, Lotz JP, Benbunan M (2001) Cytokines and vascular cell adhesion molecule-1 in the blood of patients undergoing HPC mobilization. Transfusion 41:206–212
Lévesque JP, Taamatsu Y, Nilsson SK, Haylock DN, Simmons PJ (2001) Vascular cell adhesion molecule-1 (CD 106) is cleaved by neutrophil proteases in the bone marrow following hematopoietic progenitor cell mobilisation by granulocyte colony-stimulating factor. Blood 98:1289–1297
Gee BE, Platt OS (1995). Sickle reticulocytes adhere to VCAM-1. Blood 85:268–274
Haider MZ, Raghupathy R, Azizieh F, Abdelsalam R, D'Souza TM, Adekile AD (2000) GM-CSF in sickle cell anemia patients with elevated HbF. Acta Haematol 102:140–143
Croizat H (1994) Circulating cytokines in sickle cell patients during steady state. Br J Haematol 87:592–597
Stuart MJ, Yamaja Setty BN (1999) Sickle cell acute chest syndrome: pathogenesis and rationale for treatment. Blood 94:1555–1560
Setty BNY, Stuart MJ (1996) Vascular cell adhesion molecule-1 is involved in mediating hypoxia-induced sickle red blood cell adherence to endothelium: potential role in sickle cell disease. Blood 88:2311–2320
Pieters RC, Rojer RA, Saleh AW, Saleh AE, Duits AJ (1995) Molgramostim to treat SS-sickle cell leg ulcers. Lancet 345:528
Dale GL, Alberio L (1998) Is there a correlation between raised erythropoietin and thrombotic events in sickle-cell anaemia? Lancet 352:566–567
Lard LR, Mul FP, de Haas M, Roos D, Duits AJ (1999) Neutrophil activation in sickle cell disease. J Leukoc Biol 66:411–415
Velders GA, Pruijt JF, Verzaal P, van Os R, van Kooyk Y, Figdor CG, de Kruijf EJ, Willemze R, Fibbe WE (2002) Enhancement of G-CSF-induced stem cell mobilization by antibodies against the beta 2 integrins LFA-1 and Mac-1. Blood 100:327–333
Miller ST, Sleeper LA, Pegelow CH, Enos LE, Wang WC, Weiner SJ, Wethers DL, Smith J, Kinney TR (2000) Prediction of adverse outcomes in children with sickle cell disease. N Engl J Med 342:83–89
Platt OS, Thorington BD, Brambilla DJ, Milner PF, Rosse WF, Vichinsky E, Kinney TR (1991) Pain in sickle cell disease. Rates and risk factors. N Engl J Med 325:11–16
Castro O, Brambilla DJ, Thorington B, Reindorf CA, Scott RB, Gillette P, Vera JC, Levy PS (1994) The acute chest syndrome in sickle cell disease: incidence and risk factors. The Cooperative Study of Sickle Cell Disease. Blood 84:643–649
Platt OS, Brambilla DJ, Rosse WF, Milner PF, Castro O, Steinberg MH, Klug PP (1994) Mortality in sickle cell disease. Life expectancy and risk factors for early death. N Engl J Med 330:1639–1644
Ohene-Frempong K, Weiner SJ, Sleeper LA, Miller ST, Embury S, Moohr JW, Wethers DL, Pegelow CH, Gill FM (1998) Cerebrovascular accidents in sickle cell disease: rates and risk factors. Blood 91:288–294
Acknowledgements
Contract grant sponsor: the Netherlands Antilles Society for Higher Clinical Education (NASKHO). H. ten Cate is an established investigator of the Netherlands Heart Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Duits, A.J., Rojer, R.A., van Endt, T. et al. Erythropoiesis and serum sVCAM-1 levels in adults with sickle cell disease. Ann Hematol 82, 171–174 (2003). https://doi.org/10.1007/s00277-003-0610-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-003-0610-8