Abstract
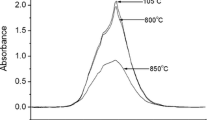
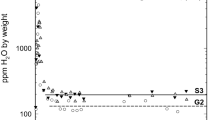
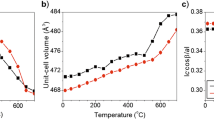
Oxidation and dehydrogenation processes for heat-treated anthophyllites were investigated using Mössbauer and infrared spectroscopy. At temperatures from 350°C to about 650°C, Fe2+ at the M1 and M3 sites oxidizes, yielding Fe3+ + one electron. A proton from the (OH)– is liberated and combines with this electron to form a hydrogen atom; and some Fe2+ ions at the M2 and M4 sites exchange with Mg at the M1 and M3 sites and then are oxidized in a similar way; at higher temperature, OH remaining in the (MgMgMg/Fe3+)-(OH)-configuration are dehydrogenated by decomposition of the amphibole to orthopyroxene and quartz. During oxidation and dehydrogenation of anthophyllite, there is disordering of Mg and Fe at the M1, M2, M3 and M4 sites in all samples studied. When all Fe2+ is oxidized, the site occupancies of at the M4 and M1, M2, M3 sites become identical, indicating that Mg and Fe3+ are completely disordered at these sites.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 12 November 1996 / Revised, accepted: 7 April 1997
Rights and permissions
About this article
Cite this article
Ishida, K. Cation disordering in heat-treated anthophyllites through oxidation and dehydrogenation. Phys Chem Min 25, 160–167 (1998). https://doi.org/10.1007/s002690050099
Issue Date:
DOI: https://doi.org/10.1007/s002690050099