Abstract
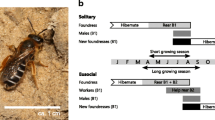
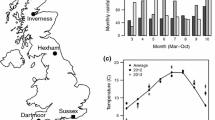
Phenotypic plasticity may evolve when conditions vary temporally or spatially on a small enough scale. Plasticity is thought to play a central role in the early stages of evolutionary transitions, including major transitions such as those between non-sociality and sociality. The sweat bee Halictus rubicundus is of special interest in this respect, because it is socially plastic in the British Isles: Nests are social or non-social depending on the environment. However, sociality comprises a complex suite of inter-related traits. To further investigate social plasticity in H. rubicundus, we measured traits that are potentially integral to social phenotype at a northern site, where nests are non-social, and a southern site where nests can be social. We found that foundresses at non-social sites were smaller, produced offspring of a size more similar to themselves, initiated nesting later, and took longer to produce their first female offspring. They began provisioning earlier in the day, finished earlier, and collected more pollen loads. Common garden experiments suggested that these differences represent mainly plasticity, as expected for traits involved in the overall plastic social phenotype, with only limited evidence for fixed genetic differences in foraging. Conditions during overwintering did not have major effects on a foundress' subsequent behaviour.






Similar content being viewed by others
References
Baglione V, Canestrari D, Marcos JM, Griesser M, Ekman J (2002) History, environment and social behaviour: experimentally induced cooperative breeding in the carrion crow. P Roy Soc Lond B Bio 269:1247–1251
Beekman M, van Stratum P, Lingeman R (1998) Diapause survival and post-diapause performance in bumblebee queens (Bombus terrestris). Entomol Exp Appl 89:207–214
Blanckenhorn WU, Demont M (2004) Bergmann and converse Bergmann latitudinal clines in arthropods: two ends of a continuum? Integr Comp Biol 44:413–424
Brady SG, Sipes S, Pearson A, Danforth BN (2006) Recent and simultaneous origins of eusociality in halictid bees. P Roy Soc Lond B Bio 273:1643–1649
Cant MA, Field J (2001) Helping effort and future fitness in cooperative animal societies. P Roy Soc Lond B Bio 268:1959–1964
Chapuisat M (2010) Evolution: plastic sociality in a sweat bee. Curr Biol 20(22):R977–R979
Conover DO, Schultz ET (1995) Phenotypic similarity and the evolutionary significance of countergradient variation. Trends Ecol Evol 10:248–252
Crawley MJ (2007) The R book. John Wiley & Sons, Chichester, UK
Cronin AL (2001) Social flexibility in a primitively social allodapine bee (Hymenoptera: Apidae): results of a translocation experiment. Oikos 94:337–343
Danforth BN (2002) Evolution of sociality in a primitively eusocial lineage of bees. P Natl Acad Sci USA 99:286–290
Eickwort GC, Eickwort JM, Gordon J, Eickwort MA (1996) Solitary behavior in a high altitude population of the social sweat bee Halictus rubicundus (Hymenoptera: Halictidae). Behav Ecol Sociobiol 38:227–233
Field J, Paxton RJ, Soro A, Bridge C (2010) Cryptic plasticity underlies a major evolutionary transition. Curr Biol 20:2028–2031
Gotthard K, Nylin S, Wiklund C (2000) Individual state controls temperature dependence in a butterfly (Lasiommata maera). P R Soc B 267:589–593
Heinrich B (1979) Bumblebee economics. Harvard University Press, Cambridge, Mass
Heinze J, Foitzik S, Fischer B, Wanke T, Kipyatkov VE (2003) The significance of latitudinal variation in body size in a holarctic ant, Leptothorax acervorum. Ecography 26:349–355
Hirata M, Higashi S (2008) Degree-day accumulation controlling allopatric and sympatric variations in the sociality of sweat bees, Lasioglossum (Evylaeus) baleicum (Hymenoptera: Halictidae). Behav Ecol Sociobiol 62:1239–1247
Honek A (1993) Intraspecific variation in body size and fecundity in insects—a general relationship. Oikos 66:483–492
Kamm DR (1974) Effects of temperature, day length, and number of adults on the sizes of cells and offspring in a primitively social bee (Hymenoptera: Halictidae). J Kansas Entomol Soc 47:8–18
Lunn DJ, Thomas A, Best N, Spiegelhalter D (2000) WinBUGS—a Bayesian modelling framework: concepts, structure, and extensibility. Stat Comput 10:325–337
Martin TG, Wintle BA, Rhodes JR, Kuhnert PM, Field SA, Low-Choy SJ, Tyre AJ, Possingham HP (2005) Zero tolerance ecology: improving ecological inference by modelling the source of zero observations. Ecol Lett 8:1235–1246
Nylin S, Gotthard K (1998) Plasticity in life-history traits. Annu Rev Entomol 43:63–83
Nylin S, Wickman PO, Wiklund C (1989) Seasonal plasticity in growth and development of the speckled wood butterfly, Pararge aegeria (Satyrinae). Biol J Linn Soc 38:155–171
Packer L, Knerer G (1985) Social evolution and its correlates in bees of the subgenus Evylaeus (Hymenoptera, Halictidae). Behav Ecol Sociobiol 17:143–149
Plateaux-Quenu C, Plateaux L, Packer L (2000) Population-typical behaviours are retained when eusocial and non-eusocial forms of Evylaeus albipes (F.) (Hymenoptera, Halictidae) are reared simultaneously in the laboratory. Insect Soc 47:263–270
R Development Core Team (2011) R: a language and environment for statistical computing. R Foundation for Statistical Computing Vienna, Austria
Reeve HK, Ratnieks FLW (1993) Queen–queen conflicts in polygynous societies: mutual tolerance and reproductive skew. In: Keller L (ed) Queen number and sociality in insects. Oxford University Press, Oxford, pp 45–85
Richards MH, Packer L (1996) The socioecology of body size variation in the primitively eusocial sweat bee, Halictus ligatus (Hymenoptera: Halictidae). Oikos 77:68–76
Roff D (1980) Optimizing development time in a seasonal environment—the ups and downs of clinal variation. Oecologia 45:202–208
Smith CR, Toth AL, Suarez AV, Robinson GE (2008) Genetic and genomic analyses of the division of labour in insect societies. Nat Rev Genet 9:735–748
Soro A, Field J, Bridge C, Cardinal SC, Paxton RJ (2010) Genetic differentiation across the social transition in a socially polymorphic sweat bee, Halictus rubicundus. Mol Ecol 19(16):3351–3363
Soucy SL (2002) Nesting biology and socially polymorphic behavior of the sweat bee Halictus rubicundus (Hymenoptera: Halictidae). Ann Entomol Soc Am 95:57–65
Soucy SL, Danforth BN (2002) Phylogeography of the socially polymorphic sweat bee Halictus rubicundus (Hymenoptera: Halictidae). Evolution 56:330–341
Strassmann JE, Queller DC (2007) Insect societies as divided organisms: the complexities of purpose and cross-purpose. Proc Nat Acad Sci U S A 104:8619–8626
Strohm E, Liebig J (2008) Why are so many bees but so few digger wasps social? The effect of provisioning mode and helper efficiency on the distribution of sociality among the Apoidea. In: Korb JH, Heinze J (eds) Ecology of social evolution. Springer, Berlin, pp 109–127
Strohm E, Marliani A (2002) The cost of parental care: prey hunting in a digger wasp. Behav Ecol 13:52–58
Weissel N, Mitesser O, Liebig J, Poethke HJ, Strohm E (2006) The influence of soil temperature on the nesting cycle of the halictid bee Lasioglossum malachurum. Insect Soc 53(4):390–398
Weissel N, Mitesser O, Poethke HJ, Strohm E (2012) Availability and depletion of fat reserves in halictid foundress queens with a focus on solitary nest founding. Insect Soc 59:67–74
West-Eberhard MJ (2003) Developmental plasticity and evolution. Oxford University Press, Oxford
Whitman DW, Agrawal AA (2009) What is phenotypic plasticity and why is it important? In: Whitman DWA, Ananthakrishna TN (eds) Phenotypic plasticity of insects: mechanisms and consequences. Science Publishers, Enfield, New Hampshire, USA, pp 1–63
Willmer PG (1985) Size effects on hygrothermal balance and foraging patterns in a sphecid wasp (Cerceris). Ecological Entomology 10:469–479
Yanega D (1989) Caste determination and differential diapause within the first brood of Halictus rubicundus in New York (Hymenoptera: Halictidae). Behav Ecol Sociobiol 24:97–107
Yanega D (1990) Philopatry and nest founding in a primitively social bee, Halictus rubicundus. Behav Ecol Sociobiol 27:37–42
Yanega D (1996) Sex ratio and sex allocation in sweat bees (Hymenoptera: Halictidae). J Kansas Entomol Soc 69:98–115
Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM (2009) Mixed effects models and extensions in ecology with R. Springer, Berlin
Acknowledgments
We thank A. De Palma, A. Eriksson and J. Green for help with field observations, and A. Cronin, J. Green, S. Kocher, E. Lucas and two anonymous referees for commenting on the manuscript. K. Henderson, A. Broadbent, M. MacDonald N. Robinson and S. Roberts helped identify the field sites. We also thank Tara Martin for the code from which we adapted the ZIP models used here. Natural England, The National Parks and Wildlife Service, Belfast City Council (Parks and Leisure Service) and individual landowners kindly gave permission for transplants and fieldwork. Funded by a Natural Environment Research Council grant to J.F and R.P. The authors declare that they have no conflict of interest. All of the UK guidelines and legal requirements for the use of animals in research were followed.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Heinze
Rights and permissions
About this article
Cite this article
Field, J., Paxton, R., Soro, A. et al. Body size, demography and foraging in a socially plastic sweat bee: a common garden experiment. Behav Ecol Sociobiol 66, 743–756 (2012). https://doi.org/10.1007/s00265-012-1322-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-012-1322-7