Abstract
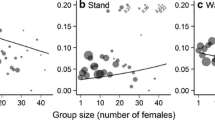
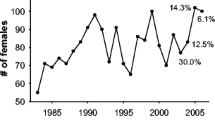
In polygynous species, males devote considerable effort to reproduction during the rut. Both the number of females in the mating group and the ratio of sexually mature males to sexually mature females [adult sex ratio (ASR)] are expected to affect the amount of effort a male devotes to reproductive activities. We predicted the reproductive effort of dominant male reindeer, measured as relative mass loss, proportions of active reproductive behaviors, and frequencies of agonistic behaviors would (1) increase with an increasing number of females in the mating group and eventually level off, and (2) exhibit a dome shape with respect to ASR in the mating group. We tested these predictions using 12 years of data collected from semi-domesticated reindeer in northern Finland. We found a positive relationship between relative mass loss and the mean number of females in the mating group for mature, but not young males. The relationship between the proportion of active reproductive behaviors performed by mature males and the mean number of females in the group was quadratic while agonistic behaviors of mature males increased with the increasing female group size. We also found that active reproductive behaviors decreased with a rising mating group ASR for mature males; whereas, young males performed more agonistic behaviors as group ASR increased. Our results point to age-specific patterns of mass loss and activity during the mating season. They also indicate that both the number of females and ASR in the mating group are important factors in determining the level of reproductive effort of dominant male reindeer.



Similar content being viewed by others
References
Abell AJ (2000) Costs of reproduction in male lizards, Sceloporus virgatus. Oikos 88:630–640
Andersson M (1994) Sexual selection. Princeton University Press, Princeton
Apollonio M, Di Vittorio I (2004) Feeding and reproductive behaviour in fallow bucks (Dama dama). Naturwissenschaften 91:579–584
Apollonio M, Festa-Bianchet M, Mari F (1989) Correlates of copulatory success in a fallow deer lek. Behav Ecol Sociobiol 25:89–97
Bartos L, Fricova B, Bartosova-Vichova J, Panama J, Sustr P, Smidova E (2007) Estimation of the probability of fighting in fallow deer (Dama dama) during the rut. Aggress Behav 33:7–13
Brown JL (1964) The evolution of diversity in avian territorial systems. Wilson Bull 76:160–169
Buschhaus NL, Lagory KE, Taylor DH (1990) Behaviour in an introduced population of fallow deer during the rut. Am Midl Nat 124:318–329
Byers JA (1997) American pronghorn: social adaptations & the ghosts of predators past. The University of Chicago Press, Chicago
Clutton-Brock TH (1989) Mammalian mating systems. Proc R Soc B: Biol Sci 236:339–372
Clutton-Brock TH, Guinness FE, Albon SD (1982) Red deer: behavior and ecology of two sexes. The University of Chicago Press, Chicago
Clutton-Brock TH, McComb KE, Deutsch JC (1996) Multiple factors affect the distribution of females in lek-breeding ungulates: a rejoinder. Behav Ecol 7:373–378
Clutton-Brock TH, Hodge SJ, Flower TP (2008) Group size and the suppression of subordinate reproduction in Kalahari meerkats. Anim Behav 76:689–700
Dubois F, Giraldeau LA, Grant JWA (2003) Resource defense in a group-foraging context. Behav Ecol 14:2–9
Emlen ST, Oring LW (1977) Ecology, sexual selection, and the evolution of mating systems. Science 197:215–223
Epsmark Y (1964) Rutting behaviour in reindeer (Rangifer tarandus). Anim Behav 12:159–163
Forsyth DM, Duncan RP, Tustin KG, Gaillard JM (2005) A substantial energetic cost to male reproduction in a sexually dimorphic ungulate. Ecology 86:2154–2163
Galimberti F, Sanvito S, Braschi C, Boitani L (2007) The cost of success: reproductive effort in male southern elephant seals (Mirounga leonina). Behav Ecol Sociobiol 62:159–171
Grant JWA, Gaboury CL, Levitt HL (2000) Competitor-to-resource ratio, a general formulation of operational sex ratio, as a predictor of competetive aggression in Japanese medaka (Pisces: Oryziidae). Behav Ecol 11:670–675
Hogg JT, Forbes SH (1997) Mating in bighorn sheep: frequent male reproduction via a high-risk “unconventional” tactic. Behav Ecol Sociobiol 41:33–48
Holand Ø, Weladji RB, Røed KH, Gjøstein H, Kumpula J, Gaillard JM, Smith ME, Nieminen M (2006) Male age structure influences females’ mass change during rut in a polygynous ungulate: the reindeer (Rangifer tarandus). Behav Ecol Sociobiol 59:682
Huber S, Millesi E, Dittami JP (2002) Paternal effort and its relation to mating success in the European ground squirrel. Anim Behav 63:157–164
Isvaran K (2005) Variation in male mating behaviour within ungulate populations: patterns and processes. Curr Sci 89:1192–1199
Jennings DJ, Carlin CM, Hayden TJ, Gammell MP (2010) Investment in fighting in relation to body condition, age and dominance rank in the male fallow deer, Dama dama. Anim Behav 79:1293–1300
Kojola I (1991) Influence of age on the reproductive effort of male reindeer. J Mammal 72:208–210
Komers PE, Pelabon C, Stenstrom D (1997) Age at first reproduction in male fallow deer: age-specific versus dominance-specific behaviors. Behav Ecol 8:456–462
Le Roux A, Cherry MI, Manser MB (2008) The audience effect in a facultatively social mammal, the yellow mongoose, Cynictis penicillata. Anim Behav 75:943–949
Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O (2009) SAS for mixed models. SAS Institute Inc, Cary
Machlis L, Dodd PWD, Fentress JC (1985) The pooling fallacy—problems arising when individuals contribute more than one observation to the data set. Z Tierpsychol 68:201–214
Mainguy J, Côté SD (2008) Age- and state-dependent reproductive effort in male mountain goats, Oreamnos americanus. Behav Ecol Sociobiol 62:935–943
Martin P, Bateson P (2007) Measuring behaviour: an introductory guide, 3rd edn. Cambridge University Press, Cambridge
Mattiangeli V, Mattiello S, Verga M (1999) The fighting technique of male fallow deer (Dama dama): an analysis of agonistic interactions during the rut. J Zool 249:339–346
Michener GR, McLean IG (1996) Reproductive behaviour and operational sex ratio in Richardson’s ground squirrels. Anim Behav 52:743–758
Mysterud A, Holand O, Roed KH, Gjostein H, Kumpula J, Nieminen M (2003) Effects of age, density and sex ratio on reproductive effort in male reindeer (Rangifer tarandus). J Zool 261:341–344
Mysterud A, Langvatn R, Stenseth NC (2004) Patterns of reproductive effort in male ungulates. J Zool 264:209–215
Mysterud A, Solberg EJ, Yoccoz NG (2005) Ageing and reproductive effort in male moose under variable levels of intrasexual competition. J Anim Ecol 74:742–754
Mysterud A, Bonenfant C, Loe LE, Langvatn R, Yoccoz NG, Stenseth NC (2008) Age-specific feeding cessation in male red deer during the rut. J Zool 275:407–412
Mysterud A, Roed KH, Holand O, Yoccoz NG, Nieminen M (2009) Age-related gestation length adjustment in a large iteroparous mammal at northern latitude. J Anim Ecol 78:1002–1006
Pelletier F, Hogg JT, Festa-Bianchet M (2006) Male mating effort in a polygynous ungulate. Behav Ecol Sociobiol 60:645–654
Pelletier F, Mainguy J, Côté SD (2009) Rut-induced hypophagia in male bighorn sheep and mountain goats: foraging under time budget constraints. Ethology 115:141–151
Pepin D, Morellet N, Goulard M (2009) Seasonal and daily walking activity patterns of free-ranging adult red deer (Cervus elaphus) at the individual level. Eur J Wildl Res 55:479–486
Roed KH, Holand O, Smith ME, Gjostein H, Kumpula J, Nieminen M (2002) Reproductive success in reindeer males in a herd with varying sex ratio. Mol Ecol 11:1239–1243
SAS (2008) The SAS system for Windows, release 9.2. In. SAS Institute Inc. Carey, North Carolina, USA
Skogland T (1989) Comparative social organization of wild reindeer in relation to food, mates and predator avoidance. Adv Ethol 29:1–77
Stearns SC (1992) The evolution of life histories. Oxford University Press, Oxford
Trivers RL (1972) Parental investment and sexual selection. In: Campbell B (ed) Sexual selection and the descent of man. Aldine, Chicago
Willisch CS, Ingold P (2007) Feeding or resting? The strategy of rutting male Alpine chamois. Ethology 113:97–104
Acknowledgements
We gratefully acknowledge the financial support of the Natural Sciences and Engineering Research Council of Canada (scholarship to E.M.T and research grant to R.B.W) and the Northern Scientific Training Program. Thanks also to J. Grant, M. Festa-Bianchet, D. Forsyth, and an anonymous referee for comments that improved this manuscript. We thank all students, assistants, and volunteers who have contributed to our research over the past 14 years. We are grateful to the Kutuharju Field Reindeer Station (Kaamanen, Finland), to Mikka Tervonen, and Heilki Tormanen for the assistance and logistic support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: M. Festa-Bianchet
Rights and permissions
About this article
Cite this article
Tennenhouse, E.M., Weladji, R.B., Holand, Ø. et al. Mating group composition influences somatic costs and activity in rutting dominant male reindeer (Rangifer tarandus). Behav Ecol Sociobiol 65, 287–295 (2011). https://doi.org/10.1007/s00265-010-1043-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-010-1043-8