Abstract
Purpose
This study evaluated the feasibility of computed magnetic resonance imaging (MRI) volumetry in conventional osteosarcomas. Secondly, we investigated whether computed volumetry provides new prognostic indicators for histological response of osteosarcomas after neoadjuvant chemotherapy.
Methods
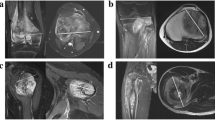
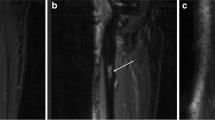
In a retrospective cohort study, data from the Vienna Bone Tumour Registry was used. MR images from 14 patients (male:female = 1.8, mean age 19 years) were analysed prior to and after neoadjuvant chemotherapy according to current therapy regimens. Histological response to chemotherapy was graded according to the Salzer-Kuntschik classification. Computed volumetry was performed for the intraosseous part, as well as the soft-tissue component and the tumour as a whole.
Results
In a setting of appropriate radiological equipment, the method has been considered to be well implementable into clinical routine. The mean tumour volume prior to chemotherapy was 321 (±351) ml. In good responders (n = 6), overall tumour volume decreased by 47 % (p = 0.345), whereas poor responders (n = 8) showed a 19 % decrease (p = 0.128). Neoadjuvant multidrug therapy remarkably changed the tumour composition. This is seen in a decrease of the mean ratio of soft-tissue to intraosseous tumour volume from 8.67 in poor responders and 1.15 in good responders to 1.26 and 0.45 (p = 0.065), respectively. Interestingly, the bony compartment of good responders showed a volume increase during neoadjuvant chemotherapy (p = 0.073). However, we did not find prognostic markers for histological tumour response to pre-operative chemotherapy.
Conclusions
Separated volumetry of tumour segments revealed interesting insights into therapy-induced growth patterns. If verified in a larger study population, these results should be taken into account when planning ablative surgery.



Similar content being viewed by others
References
Fletcher C, Unni K, Mertens F (2002) World Health Organization classification of tumours. Pathology and genetics of tumours of soft tissue and bone. IARC Press, Lyon, pp 264–284
Carrle D, Bielack SS (2006) Current strategies of chemotherapy in osteosarcoma. Int Orthop 30:445–451. doi:10.1007/s00264-006-0192-x
Jaffe N (2010) Osteosarcoma: review of the past, impact on the future. The american experience. Cancer Treat Res 152:239–262. doi:10.1007/978-1-4419-0284-9_12
Li X, Moretti VM, Ashana AO, Lackman RD (2012) Impact of close surgical margin on local recurrence and survival in osteosarcoma. Int Orthop 36:131–137. doi:10.1007/s00264-011-1230-x
Hayashi K, Tsuchiya H, Yamamoto N et al (2008) Functional outcome in patients with osteosarcoma around the knee joint treated by minimised surgery. Int Orthop 32:63–68. doi:10.1007/s00264-006-0289-2
Marina N, Bielack S, Whelan J et al (2010) International collaboration is feasible in trials for rare conditions: the EURAMOS experience. Cancer Treat Res 152:339–353. doi:10.1007/978-1-4419-0284-9_18
Funovics PT, Edelhauser G, Funovics MA et al (2011) Pre-operative serum C-reactive protein as independent prognostic factor for survival but not infection in patients with high-grade osteosarcoma. Int Orthop 35:1529–1536. doi:10.1007/s00264-011-1208-8
Rastogi S, Kumar R, Sankineani SR et al (2012) Role of vascular endothelial growth factor as a tumour marker in osteosarcoma: a prospective study. Int Orthop 36:2315–2321. doi:10.1007/s00264-012-1663-x
Davis AM, Wright JG, Williams JI et al (1996) Development of a measure of physical function for patients with bone and soft tissue sarcoma. Qual Life Res 5:508–516
Picci P, Sangiorgi L, Rougraff BT et al (1994) Relationship of chemotherapy-induced necrosis and surgical margins to local recurrence in osteosarcoma. J Clin Oncol 12:2699–2705
Salzer-Kuntschik M, Delling G, Beron GR (1983) Morphological grades of regression in osteosarcoma after polychemotherapy—study COSS 80. J Cancer 106:21–24
Bajpai J, Gamnagatti S, Kumar R et al (2011) Role of MRI in osteosarcoma for evaluation and prediction of chemotherapy response: correlation with histological necrosis. Pediatr Radiol 41:441–450. doi:10.1007/s00247-010-1876-3
Bacci G, Lari S (2001) Adjuvant and neoadjuvant chemotherapy in osteosarcoma. Chir Organi Mov 86:253–268
Kong C, Hansen M (2009) Biomarkers in osteosarcoma. Expert Opin Med 3:13–23. doi:10.1517/17530050802608496.Biomarkers
Bieling P, Rehan N, Winkler P et al (1996) Tumor size and prognosis in aggressively treated osteosarcoma. J Clin Oncol 14:848–858
Kim MS, Lee S-Y, Cho WH et al (2008) Initial tumor size predicts histologic response and survival in localized osteosarcoma patients. J Surg Oncol 97:456–461. doi:10.1002/jso.20986
Holscher H, Bloem J, Van der Woude H et al (1995) Can MRI predict the histopathological response in patients with osteosarcoma after the first cycle of chemotherapy? Clin Radiol 50:384–390
Bielack S, Jürgens H, Jundt G et al (2010) Osteosarcoma: the COSS experience. Cancer Treat Res 152:289–308. doi:10.1007/978-1-4419-0284-9_15
Denecke T, Hundsdörfer P, Misch D et al (2010) Assessment of histological response of paediatric bone sarcomas using FDG PET in comparison to morphological volume measurement and standardized MRI parameters. Eur J Nucl Med Mol Imaging 37:1842–1853. doi:10.1007/s00259-010-1484-3
Schima W, Amann G, Stiglbauer R et al (1994) Preoperative staging of osteosarcoma: efficacy of MR imaging in detecting joint involvement. AJR Am J Roentgenol 163:1171–1175
Hoffer FA, Nikanorov AY, Reddick WE et al (2000) Accuracy of MR imaging for detecting epiphyseal extension of osteosarcoma. Pediatr Radiol 30:289–298
Masrouha KZ, Musallam KM, Samra AB et al (2012) Correlation of non-mass-like abnormal MR signal intensity with pathological findings surrounding pediatric osteosarcoma and Ewing’s sarcoma. Skeletal Radiol 41:1453-1461. doi:10.1007/s00256-012-1383-8
James SLJ, Panicek DM, Davies AM (2008) Bone marrow oedema associated with benign and malignant bone tumours. Eur J Radiol 67:11–21. doi:10.1016/j.ejrad.2008.01.052
Yamamura S, Sato K, Sugiura H et al (1997) Prostaglandin levels of primary bone tumor tissues correlate with peritumoral edema demonstrated by magnetic resonance imaging. Cancer 79:255–261
Shinmura K, Ishida T, Goto T et al (2004) Expression of cyclooxygenase-2 in chondroblastoma: immunohistochemical analysis with special emphasis on local inflammatory reaction. Virchows Arch 444:28–35. doi:10.1007/s00428-003-0889-9
White LM, Wunder JS, Bell RS et al (2005) Histologic assessment of peritumoral edema in soft tissue sarcoma. Int J Radiat Oncol Biol Phys 61:1439–1445. doi:10.1016/j.ijrobp.2004.08.036
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Laux, C.J., Berzaczy, G., Weber, M. et al. Tumour response of osteosarcoma to neoadjuvant chemotherapy evaluated by magnetic resonance imaging as prognostic factor for outcome. International Orthopaedics (SICOT) 39, 97–104 (2015). https://doi.org/10.1007/s00264-014-2606-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-014-2606-5