Abstract
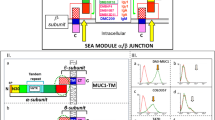
A recombinant diabody fragment based on the anti-MUC1 monoclonal antibody, C595 has been produced in a bacterial expression system. Substitution of a 7-amino-acid linker sequence (Gly6Ser) for the original single-chain (sc)Fv 15-amino-acid linker (Gly4Ser)3, using polymerase-chain-reaction-based strategies, forces variable heavy (VH) and light (VL) domains to pair with complementary domains on neighbouring scFv molecules, forming a scFv dimer (diabody). This recombinant protein shows similar binding characteristics to the parental C595 monoclonal antibody. The ability to bind to MUC1 mucin on carcinoma cell surfaces will allow its potential as a diagnostic and therapeutic reagent of clinical utility to be investigated.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 16 September 1998 / Accepted: 2 December 1998
Rights and permissions
About this article
Cite this article
Denton, G., Brady, K., Lo, B. et al. Production and characterization of an anti-(MUC1 mucin) recombinant diabody. Cancer Immunol Immunother 48, 29–38 (1999). https://doi.org/10.1007/s002620050545
Issue Date:
DOI: https://doi.org/10.1007/s002620050545