Abstract
T cells expressing a mesothelin (MSLN)-specific T cell receptor fusion construct (TRuC®), called TC-210, have demonstrated robust antitumor activity in preclinical models of mesothelioma, ovarian cancer, and lung cancer. However, they are susceptible to suppression by the programmed cell death protein 1 (PD-1)/programmed cell death protein ligand 1 (PD-L1) axis and lack intrinsic costimulatory signaling elements. To enhance the function of anti-MSLN TRuC-T cells, chimeric switch receptors (CSRs) have been designed to co-opt the immunosuppressive PD-1/PD-L1 axis and to deliver a CD28-mediated costimulatory signal. Here, we report that coexpression of the PD1-CD28 CSR in TRuC-T cells enhanced T cell receptor signaling, increased proinflammatory effector cytokines, decreased anti-inflammatory cytokines, and sustained effector function in the presence of PD-L1 when compared with TC-210. Anti-MSLN TRuC-T cells engineered to coexpress PD1-CD28 CSRs comprising the ectodomain of PD-1 and the intracellular domain of CD28 linked by the transmembrane domain of PD-1 were selected for integration into an anti-MSLN TRuC-T cell therapy product called TC-510. In vitro, TC-510 showed significant improvements in persistence and resistance to exhaustion upon chronic stimulation by tumor cells expressing MSLN and PD-L1 when compared with TC-210. In vivo, TC-510 showed a superior ability to provide durable protection following tumor rechallenge, versus TC-210. These data demonstrate that integration of a PD1-CD28 CSR into TRuC-T cells improves effector function, resistance to exhaustion, and prolongs persistence. Based on these findings, TC-510 is currently being evaluated in patients with MSLN-expressing solid tumors.
Similar content being viewed by others
Introduction
We have engineered a novel adoptive T cell therapy platform by integrating a T cell receptor fusion construct (TRuC®) into the T cell receptor (TCR). This leverages the full signaling capacity of the TCR in a human leukocyte antigen (HLA)-independent manner and addresses the limitations of chimeric antigen receptor engineered T (CAR-T) cells and TCR-engineered T (TCR-T) approaches. TRuC-T cells consist of a tumor antigen binding domain fused to the CD3ε subunit of the TCR complex, which upon its integration into the TCR redirects T cell killing against tumor cells. This novel design has shown functional advantages over CAR-T cells in preclinical models, including faster tumor regression, lower cytokine production, increased solid tumor infiltration, increased oxidative metabolism, and enhanced persistence [1]. Based on promising preclinical evidence, a Phase 1/2 clinical trial examining TC-210 (gavocabtagene autoleucel [gavo-cel]) is ongoing in patients with advanced mesothelin (MSLN)-expressing cancer (NCT03907852).
The tumor microenvironment (TME) of solid tumors presents a major hurdle in realizing the full potential of T cell therapies, as immunoinhibitory molecules are often abundant, and positive costimulatory molecules are lacking [2]. Overexpression of programmed cell death ligand (PD-L)1 and PD-L2 on tumor cells directly inhibits T cell function by activating the programmed cell death protein 1 (PD-1) [3].
Furthermore, full T cell activation requires TCR recognition of cognate peptide major histocompatibility complexes (MHCs) (signal 1) in conjunction with costimulation, driven most prominently by activation of CD28 (signal 2) [4]. A lack of sufficient costimulatory signaling leads TCR-activated T cells to enter a hyporesponsive state known as anergy [5].
Suppression by the PD-1/PD-L1 axis-mediated suppression within the TME and the lack of intrinsic CD28 signaling afforded by the TRuC construct may present hurdles to optimal TRuC-T cell efficacy. Thus, we engineered and preclinically tested chimeric switch receptors (CSRs) designed to co-opt the immunosuppressive PD-1/PD-L1 axis and, at the same time, deliver a CD28-mediated costimulatory signal. CSRs are designed to convert a normally immunoinhibitory interaction into an immunostimulatory event by genetically linking the extracellular domain of a suppressive receptor (in this case PD-1) to the signaling domain of an activating receptor (in this case CD28).
We benchmarked the activity of anti-MSLN TRuC-T cells coexpressing CSRs to T cells bearing only the anti-MSLN TRuC (TC-210 T cells). As it has been established that the transmembrane (TM) domain can influence the functionality of a chimeric receptor [6], we compared anti-MSLN TRuC-T cells engineered to coexpress PD1-CD28 CSRs containing either PD1TM or CD28TM. The resulting lead construct, PD1TM CSR, was integrated into a novel anti-MSLN cell therapy product designated TC-510. The efficacy of TC-510 and TC-210 were compared using an in vitro stimulation assay. Durable protection against tumor rechallenge in vivo was also assessed.
Materials and methods
T cell engineering
MSLN-targeting ɛ-TRuC was generated as described [1]. A PD1TM CSR was generated by isothermal assembly of the ecto- and TM domains of PD-1 (Q15116 amino acids 1–191) to the intracellular domain of CD28 (P10747 amino acids 180–220). Similarly, a CD28TM CSR was generated by isothermal assembly of the ectodomain of PD-1 (Q15116 amino acids 1–170) to the TM and intracellular domains of CD28 (P10747 amino acid 153–220). MSLN-targeting ɛ-TRuC and the CSR were cloned on the same lentivirus expression vector upstream and downstream of a T2A sequence, respectively.
Lentiviruses were prepared by transient transfection of HEK293 suspension cells with packaging plasmids and the TRuC or CAR lentiviral transfer plasmids. Supernatants were collected 48 h post-transfection, centrifuged, filtered, and precipitated. Clarified supernatants were resuspended in TexMACS medium (Miltenyi Biotech, Berisch Gladbacj, Germany) supplemented with 3% human antibody serum (Gemini Bio-Products, West Sacramento, CA) and stored at –80°C until use.
On Day 0, primary human T cells were isolated by magnetic bead separation using anti-CD4 and anti-CD8 microbeads. T cells were activated using Human T Cell TransAct (Miltenyi Biotech) at a 1:1 ratio and cultured in TexMACS medium with 3% human antibody serum (Gemini Bio-Products), 12.5 ng/mL human IL-7, and 12.5 ng/mL human IL-15 (Miltenyi Biotech). T cells were transduced with the respective lentiviral vectors on Day 1, harvested on Day 10, and frozen prior to use in functional assays.
Cell lines
Tumor cell lines were purchased from ATCC (mesothelioma [MSTO]-211H [CRL-2081™]; Mannasas, VA) or Millipore Sigma (A2780 [C30]; St Louis, MO). For the generation of target cell lines, full-length firefly luciferase (Luc) or the PD-L1 ecto- and TM domains were cloned into pCDH-CMV-MCS-EF1a-Neo. Full-length human MSLN was cloned into pCDH- pCDH-EF1a-MCS-T2A-Puro (SBI, Palo Alto, CA), using XbaI and EcoRI restriction sites. Stably transduced cells were selected with neomycin (Millipore Sigma) and/or puromycin (Corning, Bedford, MA).
Flow cytometry analysis
The transduction efficiency, in vitro expansion, activation/exhaustion, and proliferation of engineered T cells were analyzed by flow cytometric analysis. Cells were stained using fluorescently labeled antibody cocktails, and data were acquired on the BD LSR Fortessa™ X-20 cell analyzer. Data analysis was performed using FlowJo software (TreeStar Inc, Ashland, OR). Detailed methods are provided in the supplemental material.
Luciferase activity-based tumor cell cytotoxicity assay
Luciferase-expressing tumor cells were plated in triplicate in a 96-well plate at 1.0 × 104 cells per well, and T cells were added at the desired effector-to-target (E-to-T) ratios. After 24-h coculture, 50% of the culture supernatant was removed for cytokine analysis. Cell viability was determined using the Bright-Glo™ Luciferase Assay System (Promega, Madison, WI) according to the manufacturer’s protocol. Relative luminescence units (RLU) were measured using the SpectraMax M5 plate reader (Molecular Devices, Sunnyvale, CA). The percentage of tumor cell killing was calculated by the following formula: % tumor cell lysis = 100% × [(1 − RLU (tumor cells + T cells)/RLU (tumor cells)].
Coculture assays
For TRuC-T cell coculture assays with target cell lines, TRuC-T cells were first thawed and rested in IL-2 (300 U/mL) for 72 h. At the end of the rest period, TRuC-T cells were then normalized for transduction efficiency and then plated in a 96-well U-bottom plate at a 1:1 ratio with 1.0 × 105 Streck-treated tumor cells (Streck, La Vista, NE) for up to 96 h. Culture supernatants were harvested from replicate plates at 24 or 72 h and stored at –80 °C until sample analysis. Detailed methods, including rechallenge assay conditions, are provided in the supplemental material.
Plate-bound MSLN and PD-L1 assay
TRuC-T cells were recovered from cryopreservation by incubation in IL-2 (300 IU/mL) for 72 h. MSLN- and PD-L1-coated 96-well ELISA microplates were prepared by treatment for 24 h with PD-L1-Fc alone (2 mg/mL) or MSLN (1 mg/mL) with varying concentrations of PD-L1-Fc (0–10 mg/mL), washed, and stored semi-dry prior to use. Recovered TRuC-T cells were normalized for transduction efficiency, and incubated at 1 × 105 TRuC-T cells/well in coated-plates for 72 h. The resulting levels of IL-2, IFN-γ, TNF-α, and GM-CSF were measured using a Meso-Scale Discovery gold kit (Mesoscale Diagnostics, Rockville, MD) per the manufacturer’s instructions.
In vivo efficacy of engineered T cells
For the subcutaneous xenograft model, 1.0 × 106 MSTO-MSLN-PD-L1-Luc cells were resuspended in sterile PBS, mixed 1:1 with ice cold Matrigel® (Corning, Tewksbury, MA), and then injected subcutaneously in the dorsal hind flank of 7–8-week-old female class I/class II negative NOD scid gamma (NSG) mice (NOD.Cg-Prkdcscid H-2K1tm1BpeH2-Ab1em1MvwH2D1tm1BpeIl2rgtm1Wjl/SzJ) from the Jackson Laboratory (Bar Harbor, ME). Mice were randomized into treatment groups by tumor burden prior to injection of human T cells; n = 10 mice per group. Engineered human T cells were administered at a dose of 2.0 × 106 TRuC+ T cells per mouse, via tail vein injection when the tumor size was 150–200 mm3 (Day 0). Tumor growth was monitored as tumor volume by caliper measurement twice weekly. The volume of tumor was calculated as: tumor volume = (length × width2)/2. For tumor rechallenge in mice that had become tumor-free, 1.0 × 106 MSTO-MSLN-PD-L1-Luc cells were prepared as described above and injected subcutaneously in the opposing flank on Day 44. Data represent two independent experiments with two T cell donors.
Results
Phenotype of TRuC-T cells coexpressing a PD1-CD28 CSR
Purified T cells were activated and transduced with a lentiviral construct expressing the anti-MSLN TRuC (TC-210) alone, or in combination with CSRs harboring the CD28TM or the PD1TM (Fig. 1a). After a 9-day expansion period, a similar transduction efficiency, as assessed by the total percentage of TRuC-expressing T cells, was observed between the TC-210 and TC-210 + PD1TM CSR. T cells with the CD28TM CSR showed a significantly lower transduction efficiency of the TRuC (Fig. 1b, c) and a reduction in median fluorescence intensity (MFI) for TRuC expression compared with TC-210 alone (Fig. 1d). T cells expressing PD1TM and CD28TM CSRs showed a similar level of PD-1 expression (mean MFI of 4870 for PD1TM and 4770 for CD28TM), indicating comparable levels of chimeric receptor expression, which were ~18-fold higher than the endogenous PD-1 levels in TC-210 alone (mean MFI of 268 in TC-210; Fig. 1e). The ratio of CD4+ to CD8+ T cells was significantly increased in all transduced groups in comparison with nontransduced (NT) controls, and the ratio was generally comparable across the transduced groups (Fig. 1f). All TRuC-T cell products showed predominantly comparable profiles with respect to memory phenotype (Fig. S1a), and the expression of activation and inhibition markers (Fig. S1b and S1c).
Characterization of MSLN TRuC-T cells expressing a chimeric PD1-CD28 receptor. a Lentiviral constructs containing the MH1 anti-mesothelin TRuC (TC-210), or with a bi-cistronic construct containing the anti-mesothelin TRuC followed by a sequence encoding the PD1-CD28 CSR with the transmembrane region of either PD-1 (PD1TM) or CD28 (CD28TM). b Coexpression of PD-1 and the TRuC receptor in T cells at Day 10 of expansion. c Percent transduction of CD3+ T cells as measured by TRuC receptor expression. d MFI of TRuC receptor expression of TRuC+ T cells. e MFI of PD-1 expression of TRuC+ T cells. f Frequency of CD8+ and CD4+ T cells TRuC+ T cells on Day 10 of process. Data were analyzed for statistical significance by two-way ANOVA. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Data shown are from a single experiment representing nine donors. CD cluster of differentiation, CSR chimeric switch receptor, ECD extracellular domain, ICD intracellular domain, LTR long terminal repeat, MFI median florescence intensity, MSLN mesothelin, NT nontransduced, PD-1 programmed cell death protein 1, TRuC T cell receptor fusion construct, TMD transmembrane domain
In vitro functional characterization of TC-210 T cells bearing PD1-CD28 CSRs
The in vitro antitumor response of TC-210 and TC-210 + PD1-CD28 CSRs was assessed using the mesothelioma cell line, MSTO-211H, engineered to express human MSLN (MSTOMSLN) or MSLN and PD-L1 (MSTOMSLN-PD-L1). Resting TRuC-T cells were cocultured with the MSLN-negative cell line A2780 (C30), MSTOMSLN, or MSTOMSLN-PD-L1, and cytotoxicity was assessed after 24 h. Potent, antigen-specific cytotoxicity against both MSTOMSLN and MSTOMSLN-PD-L1 was observed for all three TRuC-T cell products, with no observable differences in tumor lysis, irrespective of PD-L1 expression by the target cells (Fig. 2a).
PD1-CD28 CSR enhances the function of MSLN TRuC-T cells. a Luc-expressing tumor cell cytotoxicity based on luminescence after 24-h coculture with MSLN TRuC-T cells at various ratios. b IFN-γ, IL-2, GM-CSF, and TNF-α were measured from the culture supernatants of the cytotoxicity assay by MSD ELISA. c Cytokine release after 24 h 1:1 coculture of MSLN TRuC-T cells with MSTOMSLN, MSTOMSLN-PD-L1, or MSTOMSLN-PD-L1 cells in the presence of an anti-PD-1 monoclonal antibody (+ PD-1); IFN-γ, IL-2, GM-CSF, TNF-α, IL-17, and IL-10 levels measured by MSD ELISA. Plotted data represent two or three individual donors and are plotted as mean (± SEM). Data were analyzed for statistical significance by two-way ANOVA. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. CSR chimeric switch receptor, ELISA enzyme-linked immunosorbent assay, GM-CSF granulocyte macrophage colony-stimulating factor, IFN-γ interferon gamma, IL interleukin, MSD meso scale discovery, MSLN mesothelin, MSTO mesothelioma, PD-L1 programmed cell death protein ligand 1, SEM standard error of the mean, TNF-α tumor necrosis alpha, TRuC T cell receptor fusion construct
While equivalent in their cytotoxicity, levels of cytokine secretion differed between TC-210 alone and TC-210 + PD1-CD28 CSRs, with the latter cells displaying a significantly higher level of proinflammatory cytokine (IL-2, TNF-α, and GM-CSF) production with both MSTOMSLN and MSTOMSLN-PD-L1 target cells (Fig. 2b). Notably, the CD28TM group produced similar amounts of cytokines in response to both MSTOMSLN and MSTOMSLN-PD-L1 targets, whereas the PD1TM group produced lower IL-2, TNF-α, and GM-CSF upon stimulation with MSTOMSLN than with MSTOMSLN-PD-L1 cells, suggesting that the PD1TM group may require a higher PD-L1 density for full activation (Fig. 2b). We confirmed moderate endogenous PD-L1 expression in MSTOMSLN and high ectopic PD-L1 expression in MSTOMSLN-PD-L1 (Fig. S2), demonstrating that both PD1TM and CD28TM TRuC-T cells exhibit potent cytokine responses toward tumors with either physiological or supraphysiological levels of PD-L1 expression. To confirm that increased cytokine production was mediated by CSR engagement of PD-L1, we added a PD-1-blocking antibody to the cocultures. Blockade of the CSR/PD-L1 interaction reduced proinflammatory cytokine production by CSR-bearing TRuC-T cells at least to the levels observed for TC-210 alone (Fig. 2c).
In sum, while addition of the PD1-CD28 CSRs did not increase redirected killing of tumor cells by MSLN TRuC-T cells, we observed a higher cytokine secretion and increased TCR signaling in cells bearing the CSRs.
Regulation of PD1-CD28 CSR activation by PD-L1
We next assessed the sensitivity of the PD1-CD28 CSRs to increasing concentrations of plate-bound Fc-conjugated PD-L1 in the presence of a fixed concentration (1.0 μg/mL) of plate-bound MSLN that we determined to stimulate a moderate IFN-γ response (Fig. S3a). PD-L1 alone did not induce cytokine production by any TRuC-T cell (Fig. 3a), demonstrating the PD-1 CSRs adhere to the two-signal model of T cell activation. In the absence of PD-L1, MSLN antigen induced comparable levels of cytokine production by all tested TRuC-T cells. However, upon stimulation with MSLN and PD-L1, TRuC-T cells with PD1TM and CD28TM CSRs both showed increased cytokine production relative to TC-210, with the CD28TM groups consistently producing the highest levels of cytokines (Fig. 3a). For most of the cytokines measured, the PD1TM group showed a clear dose-dependent response to plate-bound PD-L1, whereas the CD28TM group was strongly activated, even at low PD-L1 levels. Furthermore, after 96 h of culture, the fold expansion of the CD28TM group peaked at 2.0 μg/mL of PD-L1 and then decreased at higher concentrations (Fig. S3b). In contrast, the PD1TM group continued to expand at higher concentrations of PD-L1 (Fig. S3b). This difference in fold expansion was associated with decreased viability of the CD28TM group (Fig. S3b and S3c).
Costimulation through the chimeric PD-1 receptor is regulated by PD-L1 density. a MSD ELISA results from TRuC-T cells cultured with MSLN and increasing concentrations of PD-L1-Fc. b MSD ELISA results from MSLN TRuC-T cells cultured at a 1:1 ratio with low-MSLN antigen expressing cell lines C30, C30 overexpressing PD-L1 (C30PD-L1), parental MSTO, and MSTO overexpressing PD-L1 (MSTOPD-L1). Data were analyzed for statistical significance by two-way ANOVA. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Plotted data represent two or three individual donors and are plotted as mean (± SEM). ELISA enzyme-linked immunosorbent assay, GM-CSF granulocyte macrophage colony-stimulating factor, IFN-γ interferon gamma, IL interleukin, MSD meso scale discovery, MSLN mesothelin, MSTO mesothelioma, NS non-stimulated, NT nontransduced, PD-L1 programmed cell death protein ligand 1, SEM standard error of the mean, TNF-α tumor necrosis alpha, TRuC T cell receptor fusion construct
To further compare the activation thresholds for the PD1-CD28 CSRs, we forced expression of PD-L1 in the MSLN-negative cell line C30 (C30PD−L1) and parental MSTO-211H cells (MSTOPD−L1), which express low levels of MSLN insufficient for full TRuC activation. When the MSLN TRuC-T cells were cocultured with C30 or C30PD−L1 cell lines, the CSR groups displayed a baseline response, further demonstrating the dependence of CSR activity on TRuC engagement. When the MSLN TRuC-T cells were cocultured with the parental MSTO cell line, the CD28TM group showed a significantly heightened cytokine response, that reduced to baseline when PD-L1 was over-expressed (MSTOPD−L1) (Fig. 3b). These results suggest that the CD28TM CSR sensitizes TRuC-T cells to low MSLN expression in the presence of endogenous levels of PD-L1. The heightened sensitivity of the CD28TM group for activation compared with the PD1TM group may increase the risk of cytokine release syndrome and of on-target/off-tumor toxicity. For these reasons, we selected the PD1TM CSR for integration with the anti-MSLN TRuC. We call this second-generation TRuC-T cell candidate TC-510.
The PD1-CD28 CSR enhances TC-510 TRuC-T cell persistence in vitro in a CD28 signaling dependent manner
In addition to effector function, CD28-mediated costimulation enhances both the survival and proliferation of activated T cells. To determine if the PD1-CD28 CSR enhances the fitness of TRuC-T cells, we subjected them to an in vitro tumor rechallenge assay with MSTOMSLN-PD-L1 tumor cells at a low effector-to-target ratio, followed by a rechallenge every 96 h. To determine the relative contributions of PD-1 competition and CD28 costimulation to the enhanced effector function of PD1TM TRuC-T cells, we introduced previously characterized nonfunctional mutations into the CSR (PD1TMMutant) [7] or deleted the CD28 signaling domain entirely (PD1Trunc) and verified that these constructs coexpressed well with the TRuC (Fig. S4a and S4b).
TRuC-T cells normalized for transduction efficiency showed a comparable function in response to the initial antigen exposure, with no discernible differences in expansion or cytokine production between TC-210, PD1TMMutant, and PD1Trunc cultures (Fig. 4a, b). Following the second and third rounds of stimulation, these cultures showed contraction relative to the peak at Day 4. Examination of the culture morphology prior to the third round of stimulation revealed a diffuse pattern of cells in the TC-210, PD1TMMutant, and PD1Trunc culture conditions in comparison with more defined clusters of cells in the PD1TM (TC-510) cultures (Fig. 4c). Characterization of these cultures by flow cytometry revealed that the decline in expansion and cytokine production found in TC-210, PD1TMMutant, and PD1Trunc cultures was associated with coexpression of the exhaustion markers LAG3 and TIGIT by TRuC+ T cells (Fig. 4d). In contrast, the TC-510 group displayed continuous expansion over the course of the assay (Fig. 4a), with an increased and better sustained cytokine response compared with TC-210, PD1TMMutant, and PD1Trunc (Fig. 4b). Furthermore, TC-510 showed a less exhausted phenotype at the end of the assay (Fig. 4d).
Chimeric PD-1 receptor confers enhanced fitness to TRuC-T cells during a repeated stimulation assay. a Fold expansion of TRuC-T cells normalized for transduction efficiency and cultured with MSTOMSLN-PD-L1 tumor cells at a 1:20 effector-to-target ratio. b MSD ELISA results from culture supernatants collected 72 h after each antigen challenge and analyzed for cytokines. c Brightfield microscopy images showing visible clustering in cultures containing TC-510 T cells at Day 8. d FACs plots of CD3 and TRuC receptor expression on viable CD45+ from MSTOMSLN-PD-L1 cultures on Day 12 of culture. Statistical analysis was carried out with a two-way ANOVA. *p < 0.05, ***p < 0.01, ****p < 0.0001. Data are representative of three independent donors and are plotted as mean (± SEM). ELISA enzyme-linked immunosorbent assay, GM-CSF granulocyte macrophage colony-stimulating factor, IFN-γ interferon gamma, IL interleukin, LAG-3 lymphocyte activation gene 3, MSD meso scale discovery, MSLN mesothelin, MSTO mesothelioma, Mut mutant, NT nontransduced, PD-1 programmed cell death protein 1, PD-L1 programmed cell death protein ligand 1, SEM standard error of the mean, TIGIT T cell immunoglobulin and ITIM domain, TNF-α tumor necrosis alpha, TRuC T cell receptor fusion construct
The PD1-CD28 CSR endows TC-510 T cells an ability to protect from tumor rechallenge in vivo
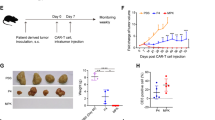
To confirm the enhanced functionality of TC-510 TRuC-T cells in an in vivo setting, MHC Class I/II null NSG mice were subcutaneously implanted with MSTOMSLN-PD-L1 cells. After 14 days, when tumors had reached a volume of 150–200 mm3, the mice received an intravenous dose of NT, TC-210, or TC-510 TRuC-T cells (Fig. 5a). Mice treated with TC-210 and TC-510 showed comparable antitumor activity, with tumor shrinkage first evident on Day 10 post infusion and complete tumor clearance seen by Day 17 (Fig. 5b). All mice treated with TC-210 or TC-510 remained tumor-free. A tumor rechallenge was performed 44 days after T cell administration, without TC-210 or TC-510 retreatment. After a transient period of initial tumor regrowth, the mice previously treated with TC-210 or TC-510 were able to clear the rechallenge tumors; however, all the TC-210 treated mice eventually experienced tumor recurrence, whereas recurrence was limited to 1/8 mice in the TC-510 group (Fig. 5b, c). TC-510 mice that rejected the rechallenge tumors showed durable protection for the remainder of the observation period (244 days post T cell administration). This durable protection from tumor rechallenge suggests that the PD1-CD28 CSR endows TC-510 TRuC-T cells with long-term functional persistence in vivo.
The chimeric receptor enhances in vivo efficacy of TRuC-T cells. a Workflow of the in vivo study design. b Mean tumor volume over time from NSG mice subcutaneously implanted with 1.0 × 106 MSTOMSLN-PD-L1 tumor cells, and then treated with 2.0 × 106 TRuC+ T cells when tumors were 150–200 mm3 (Day 0; n = 10 mice per group). On Day 44, tumor-free mice were rechallenged with 1.0 × 106 MSTOMSLN-PD-L1 tumor cells on the opposing flank. Data are representative of two independent experiments with two donors. c Individual MSTOMSLN-PD-L1 tumor growth data from NSG mice treated with TC-210 or TC-510 on Day 0 and rechallenged with tumor cells on Day 44. Statistical analysis was carried out with a two-way ANOVA. MSTO mesothelioma, NSG NOD scid gamma, NT nontransduced, PD-L1 programmed cell death protein ligand 1, TRuC T cell receptor fusion construct
Discussion
As previously shown, TRuC-T cells differ from CAR-T cells by enabling faster tumor regression, lower cytokine production, increased tumor infiltration, and a shift toward oxidative metabolism [1]. However, like native T cells, TRuC-T cells remain susceptible to inhibition by the PD-1/PD-L1 axis [8, 9]. Furthermore, whereas engineered costimulatory signals are not required for the in vivo efficacy of TRuC-T cells, in contrast to CAR-T cells [10], we hypothesized that the delivery of costimulation in conjunction with TRuC activation would enhance T cell function and persistence. In the present study, we describe a PD1-CD28 CSR that co-opts tumor PD-L1 expression and, at the same time, drives CD28 costimulation. The potential of PD1-CD28 CSRs to improve the function of engineered T cells has previously been established in preclinical models [11,12,13,14,15] and in an early clinical trial [16].
Both blocking PD-1 and enhancing CD28 signaling are attractive strategies for driving tumor-targeted T cell activity and persistence in the TME. Indeed, the approved anti-PD-1 drugs have significantly changed the standard-of-care treatment in multiple cancers, improving overall survival, progression-free survival and durability of response [17, 18]. PD-1 inhibition of T cell signaling is mediated by recruitment of SHP phosphatases that deactivate TCR signaling by targeting key kinases of the TCR and CD28 signaling pathways [19, 20], and CD28 itself is a target of PD-1-activated SHP phosphatases [21]. Regardless of PD-1-mediated inhibition, activation of CD28 by ligation to B7.1 and B7.2 on antigen-presenting cells provides a costimulatory signal that can regulate and augment endogenous TCR signaling [22,23,24]. CD28 phosphorylation potentiates T cell activation, leading to enhanced proliferation, effector function, and notably induction of proinflammatory cytokines, such as IL-2 [25,26,27].
The selection of the TM domain utilized in a chimeric receptor can have a profound effect on its function. To our knowledge, we were first to functionally compare PD1-CD28 CSRs utilizing either a PD1TM or CD28TM. Although both versions of the CSR provided a CD28 signaling enhancement to TRuC-T cells, there were potentially important functional differences between them. The CD28TM failed to discriminate between low and high PD-L1 expression density on target cells and demonstrated an exaggerated response to low levels of PD-L1 in a plate-bound assay. Furthermore, the CD28TM consistently produced more cytokines against MSTO cells that express low levels of both MLSN and PD-L1, suggesting that the CD28TM increases TRuC antigen sensitivity when PD-L1 levels are low, but not when PD-L1 levels are high. A signaling imbalance between the TRuC and CSR in the low antigen/high PD-L1 setting may explain this observation. In contrast, the PD1TM demonstrated a greater dynamic response to PD-L1 density as it exhibited lower activation compared with CD28TM when PD-L1 density was low, but rivaled or surpassed the CD28TM when the PD-L1 density was high. Moreover, the PD1TM also maintained better T cell viability at high PD-L1 expression densities in a plate-bound assay. The stochastic sensitivity of the CD28TM to PD-L1 levels may be explained by CSR homodimerization and/or heterodimerization with endogenous CD28, resulting in amplified signaling [28]. We selected the PD1TM version of the CSR for its greater sensitivity to regulation by PD-L1 levels, which we believe reduces the risk of cytokine release syndrome and on-target/off-tumor toxicity, and integrated this with the anti-MSLN TRuC utilized in TC-210 to create a second-generation TRuC-T cell product that we call TC-510.
Like TC-210, cytotoxicity and cytokine release in TC-510 cells are dependent upon TRuC engagement by MSLN. This indicates that the costimulatory activity of the PD1-CD28 CSRs adheres to the two-step model of T cell activation [29], which is an important feature in the context of off-tumor toxicity risk.
The PD1-CD28 CSR contained in TC-510 offers two potential mechanisms for TRuC-T cell enhancement: (1) acting as a PD-1 dominant-negative receptor (DNR); and (2) delivering a costimulatory signal upon PD-L1 engagement. To elucidate the relative contributions of these two mechanisms, we compared TC-510 with T cells bearing the same TRuC but with the PD1TM CSR replaced by either a truncated PD-1 lacking an intracellular signaling domain (PD1Trunc) or a PD1TM CSR in which CD28 signaling was mutationally inactivated (PD1TMMutant). TRuC-T cells coexpressing either of these constructs failed to enhance the activity of TC-510; rather, they performed comparably to TC-210. These findings support the assertion that CD28 costimulatory signaling is the primary mechanism by which the PD1TM CSR enhances TC-510 effector function. The lack of TRuC-T cell functional enhancement by the PD-1 DNR seems unexpected but is consistent with prior observations [30]; however, others have reported CAR-T cell enhancement by a PD-1 DNR [31]. This may reflect limitations in the ability of our assays to detect PD-1-mediated suppression or an intrinsic resistance of TRuC-T cells to PD-1-mediated suppression.
Our observation of enhanced expansion and persistence of TC-510 upon serial tumor rechallenge in vitro was further supported by an in vivo study in which TC-510 showed a superior ability to durably protect mice from tumor rechallenge compared with TC-210. This result indicates that integrating a PD1-CD28 CSR into TRuC-T cells enhances their capacity for improved persistence both in vitro and in vivo, which could translate into improved clinical efficacy in cancer patients with solid tumors. Understanding the potential impact of increased activation and persistence mediated by the CSR on safety will be an important focus of planned clinical studies.
Potential limitations of these preclinical studies include the artificial, nonphysiological nature of the in vitro assays used to assess T cell functionality and the use of a xenograft mouse model lacking both an intact immune system and expression of human PD-L1 or MSLN on normal tissues.
Based on these promising preclinical findings, TC-510 is currently being evaluated in a Phase 1/2 clinical trial in patients with advanced MSLN-expressing solid tumors (NCT05451849).
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article and/or its supplementary materials.
References
Ding J, Guyette S, Schrand B et al (2023) Mesothelin-targeting T cells bearing a novel T cell receptor fusion construct (TRuC) exhibit potent antitumor efficacy against solid tumors. OncoImmunology 12:2182058. https://doi.org/10.1080/2162402X.2023.2182058
Driessens G, Kline J, Gajewski TF (2009) Costimulatory and coinhibitory receptors in anti-tumor immunity. Immunol Rev 229:126–144. https://doi.org/10.1111/j.1600-065X.2009.00771.x
Han Y, Liu D, Li L (2020) PD-1/PD-L1 pathway: current researches in cancer. Am J Cancer Res 10:727–742
Beyersdorf N, Kerkau T, Hunig T (2015) CD28 co-stimulation in T-cell homeostasis: a recent perspective. Immunotargets Ther 4:111–122. https://doi.org/10.2147/ITT.S61647
Schwartz RH (2003) T cell energy. Annu Rev Immunol 21:305–334. https://doi.org/10.1146/annurev.immunol.21.120601.141110
Guo C, Wang X, Zhang H, Zhi L, Lv T, Li M, Lu C, Zhu W (2019) Structure-based rational design of a novel chimeric PD1-NKG2D receptor for natural killer cells. Mol Immunol 114:108–113. https://doi.org/10.1016/j.molimm.2019.07.009
Tian R, Wang H, Gish GD et al (2015) Combinatorial proteomic analysis of intercellular signaling applied to the CD28 T-cell costimulatory receptor. Proc Natl Acad Sci U S A 112:E1594-1603. https://doi.org/10.1073/pnas.1503286112
Riley JL (2009) PD-1 signaling in primary T cells. Immunol Rev 229:114–125. https://doi.org/10.1111/j.1600-065X.2009.00767.x
Lim AR, Rathmell WK, Rathmell JC (2020) The tumor microenvironment as a metabolic barrier to effector T cells and immunotherapy. Elife. https://doi.org/10.7554/eLife.55185
Maher J, Brentjens RJ, Gunset G, Riviere I, Sadelain M (2002) Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta/CD28 receptor. Nat Biotechnol 20:70–75. https://doi.org/10.1038/nbt0102-70
Prosser ME, Brown CE, Shami AF, Forman SJ, Jensen MC (2012) Tumor PD-L1 co-stimulates primary human CD8(+) cytotoxic T cells modified to express a PD1:CD28 chimeric receptor. Mol Immunol 51:263–272. https://doi.org/10.1016/j.molimm.2012.03.023
Ankri C, Shamalov K, Horovitz-Fried M, Mauer S, Cohen CJ (2013) Human T cells engineered to express a programmed death 1/28 costimulatory retargeting molecule display enhanced antitumor activity. J Immunol 191:4121–4129. https://doi.org/10.4049/jimmunol.1203085
Liu X, Ranganathan R, Jiang S et al (2016) A chimeric switch-receptor targeting PD1 augments the efficacy of second-generation CAR T cells in advanced solid tumors. Cancer Res 76:1578–1590. https://doi.org/10.1158/0008-5472.CAN-15-2524
Schlenker R, Olguin-Contreras LF, Leisegang M et al (2017) Chimeric PD-1:28 receptor upgrades low-avidity T cells and restores effector function of tumor-infiltrating lymphocytes for adoptive cell therapy. Cancer Res 77:3577–3590. https://doi.org/10.1158/0008-5472.CAN-16-1922
Lesch S, Nottebrock A, Rataj F, Heise C, Endres S, Kobold S (2022) PD-1-CD28 fusion protein strengthens mesothelin-specific TRuC T cells in preclinical solid tumor models. Cell Oncol (Dordr) 46:227–235. https://doi.org/10.1007/s13402-022-00747-9
Liu H, Lei W, Zhang C et al (2021) CD19-specific CAR T cells that express a PD-1/CD28 chimeric switch-receptor are effective in patients with PD-L1-positive B-cell lymphoma. Clin Cancer Res 27:473–484. https://doi.org/10.1158/1078-0432.CCR-20-1457
He X, Xu C (2020) Immune checkpoint signaling and cancer immunotherapy. Cell Res 30:660–669. https://doi.org/10.1038/s41422-020-0343-4
Ou SL, Luo J, Wei H, Qin XL, Du SY, Wang S, Jiang Q (2022) Safety and efficacy of programmed cell death 1 and programmed death ligand-1 inhibitors in the treatment of cancer: an overview of systematic reviews. Front Immunol 13:953761. https://doi.org/10.3389/fimmu.2022.953761
Gaud G, Lesourne R, Love PE (2018) Regulatory mechanisms in T cell receptor signalling. Nat Rev Immunol 18:485–497. https://doi.org/10.1038/s41577-018-0020-8
Arasanz H, Gato-Canas M, Zuazo M, Ibanez-Vea M, Breckpot K, Kochan G, Escors D (2017) PD1 signal transduction pathways in T cells. Oncotarget 8:51936–51945. https://doi.org/10.18632/oncotarget.17232
Hui E, Cheung J, Zhu J et al (2017) T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Science 355:1428–1433. https://doi.org/10.1126/science.aaf1292
Azuma M, Ito D, Yagita H, Okumura K, Phillips JH, Lanier LL, Somoza C (1993) B70 antigen is a second ligand for CTLA-4 and CD28. Nature 366:76–79. https://doi.org/10.1038/366076a0
Freeman GJ, Freedman AS, Segil JM, Lee G, Whitman JF, Nadler LM (1989) B7, a new member of the Ig superfamily with unique expression on activated and neoplastic B cells. J Immunol 143:2714–2722
Gimmi CD, Freeman GJ, Gribben JG, Sugita K, Freedman AS, Morimoto C, Nadler LM (1991) B-cell surface antigen B7 provides a costimulatory signal that induces T cells to proliferate and secrete interleukin 2. Proc Natl Acad Sci USA 88:6575–6579. https://doi.org/10.1073/pnas.88.15.6575
Esensten JH, Helou YA, Chopra G, Weiss A, Bluestone JA (2016) CD28 costimulation: from mechanism to therapy. Immunity 44:973–988. https://doi.org/10.1016/j.immuni.2016.04.020
Fife BT, Bluestone JA (2008) Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol Rev 224:166–182. https://doi.org/10.1111/j.1600-065X.2008.00662.x
Rohrs JA, Siegler EL, Wang P, Finley SD (2020) ERK activation in CAR T cells is amplified by CD28-mediated increase in CD3zeta phosphorylation. iScience 23:101023. https://doi.org/10.1016/j.isci.2020.101023
Leddon SA, Fettis MM, Abramo K, Kelly R, Oleksyn D, Miller J (2020) The CD28 transmembrane domain contains an essential dimerization motif. Front Immunol 11:1519. https://doi.org/10.3389/fimmu.2020.01519
Ledbetter JA, Imboden JB, Schieven GL, Grosmaire LS, Rabinovitch PS, Lindsten T, Thompson CB, June CH (1990) CD28 ligation in T-cell activation: evidence for two signal transduction pathways. Blood 75:1531–1539. https://doi.org/10.1182/blood.V75.7.1531.1531
Kobold S, Grassmann S, Chaloupka M et al (2015) Impact of a new fusion receptor on PD-1-mediated immunosuppression in adoptive T cell therapy. J Natl Cancer Inst 107:djv146. https://doi.org/10.1093/jnci/djv146
Cherkassky L, Morello A, Villena-Vargas J, Feng Y, Dimitrov DS, Jones DR, Sadelain M, Adusumilli PS (2016) Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition. J Clin Invest 126:3130–3144. https://doi.org/10.1172/JCI83092
DeTora L, Toroser D, Sykes A, Vanderlinden C, Plunkett F, Lane T, Hanekamp E (2022) Good publication practice (GPP) guidelines for company-sponsored biomedical research: 2022 update. Ann Intern Med 175:1298–1304. https://doi.org/10.7326/m22-1460
Acknowledgements
The authors would like to thank Seema Shah for technical assistance in the performance of in vivo studies. This study was sponsored by TCR2 Therapeutics Inc. Medical writing support, under the guidance of the authors, was provided by Macarena Ramos Gonzalez, PhD, CMC Connect, a division of IPG Health Medical Communications, in accordance with Good Publication Practice (GPP) guidelines [32]. SK is supported by the Marie-Sklodowska-Curie Program Training Network for Optimizing Adoptive T Cell Therapy of Cancer funded by the H2020 Program of the European Union (Grant 955575); by the Hector Foundation; by the International Doctoral Program i-Target: Immunotargeting of Cancer funded by the Elite Network of Bavaria; by Melanoma Research Alliance Grants 409510; by the Else Kröner-Fresenius-Stiftung; by the German Cancer Aid (to SK); by the Ernst-Jung-Stiftung; by the LMU Munich’s Institutional Strategy LMUexcellent within the framework of the German Excellence Initiative by the Go-Bio initiative; by the m4 Award of the Bavarian Ministry of Economical Affairs, by the Bundesministerium für Bildung und Forschung; by the European Research Council Grant 756017, ARMOR-T, and the ERC proof-of-concept Grant 101100460; by the German Research Foundation (DFG) (KO5055-2-1 and 510821390) by the SFB-TRR 338/1 2021-452881907; by the Wilhelm-Sander-Stiftung, by the Fritz-Bender Foundation; and by the Deutsche José-Carreras Leukämie Stiftung.
Funding
This work was supported by TCR2 Therapeutics.
Author information
Authors and Affiliations
Contributions
DM carried out the investigation, data curation, assay development, formal data analysis, methodology, supervision, and writing (review and editing). ML carried out the investigation, data curation, assay development, formal data analysis, supervision, and review/revision. AW carried out the investigation, data curation, formal analysis, and writing (review and editing). HH carried out the data curation, formal analysis, investigation, methodology, data visualization, and review and editing. PK-K designed the construct, conceptualized the study, and took part in writing (review and editing). JD carried out the methodology and writing-review and edit. SK is the inventor of the PD1-CD28 technology, provided advice on the experiments, and carried out the data interpretation, manuscript review, and editing. PAB is the inventor of TRuC technology, supported the study, carried out the drug design, and helped with manuscript writing. RH carried out the conceptualization, supervision, data visualization, and writing-review and edit. DAG carried out the conceptualization, experimental planning, data analysis, and manuscript writing/revision. RT carried out the conceptualization, methodology, supervision, data visualization, and writing/review/revision.
Corresponding author
Ethics declarations
Conflict of interest
RT, ML, AW, and JD were employees and shareholders of TCR2 Therapeutics at the time the study was conducted. RT is a current employee of Ankyra Therapeutics. ML and AW are current employees of Adaptimmune, which acquired TCR2 Therapeutics. JD is a current employee of Myeloid Therapeutics. SK is an inventor of several patents in the field of immuno-oncology, has received honoraria from BMS, GSK, Novartis, TCR2 Therapeutics, and Miltenyi Biomedicine, received license fees from Carina Biotech and TCR2 Therapeutics, and received research support from TCR2 Therapeutics for work related to this manuscript and from Arcus Bioscience, Plectonic GmbH, and Tabby Therapeutics for work unrelated to the manuscript. SK is a former shareholder of TCR2 Therapeutics, and a current shareholder of Adaptimmune. PAB is a shareholder, scientific advisor, and founder of TCR2 Therapeutics. RH is a former employee and shareholder of TCR2 Therapeutics, and a current shareholder of Adaptimmune. DAG is a shareholder at TCR2 Therapeutics. No competing interests were disclosed by DM, HH and PK-K.
Consent for publication
Consent for publication is not required for these preclinical studies.
Ethics approval and consent to participate
All animal studies were approved by the Institutional Animal Care and Use Committee of Charles River Laboratories under protocol 2021-1261. Human T cells used in these studies were sourced commercially from healthy volunteer donors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
McCarthy, D., Lofgren, M., Watt, A. et al. Functional enhancement of mesothelin-targeted TRuC-T cells by a PD1-CD28 chimeric switch receptor. Cancer Immunol Immunother 72, 4195–4207 (2023). https://doi.org/10.1007/s00262-023-03556-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-023-03556-7