Abstract
Background
The clinical implications of the third dose of coronavirus disease 2019 (COVID-19) vaccines in patients receiving immune checkpoint inhibitors are currently unknown. We performed a prospective analysis of the Vax-On-Third study to investigate the effects of antibody response on immune-related adverse events (irAEs) and disease outcomes.
Methods
Recipients of the booster dose of SARS-CoV-2 mRNA-BNT162b2 vaccine who had received at least one course of an anti-PD-1/PD-L1 treatment before vaccination for an advanced solid malignancy were eligible.
Results
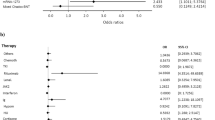
The current analysis included 56 patients with metastatic disease (median age: 66 years; male: 71%), most of whom had a lung cancer diagnosis and were being treated with pembrolizumab- or nivolumab-based regimens. The optimal cut-point antibody titer of 486 BAU/mL allowed a dichotomization of recipients into low-responders (Low-R, < 486 BAU/mL) or high-responders (High-R, ≥ 486 BAU/mL). After a median follow-up time of 226 days, 21.4% of patients experienced moderate to severe irAEs without any recrudescence of immune toxicities preceding the booster dose. The frequencies of irAE before and after the third dose did not differ, but an increase in the cumulative incidence of immuno-related thyroiditis was observed within the High-R subgroup. On multivariate analysis, an enhanced humoral response correlated with a better outcome in terms of durable clinical benefit, which resulted in a significant reduction in the risk of disease control loss but not mortality.
Conclusions
Our findings would strengthen the recommendation not to change anti-PD-1/PD-L1 treatment plans based on current or future immunization schedules, implying that all these patients should be closely monitored.





Similar content being viewed by others
Data availability
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
References
Daher A, El Zarif T, Friese CR, Griffiths EA, Hawley JE, Hayes-Lattin B, Karivedu V, Latif T, Mavromatis BH, McKay RR, Nagaraj G, Nguyen RH, Panagiotou OA, Portuguese AJ, Puc M, Santos Dutra M, Schroeder BA, Thakkar A, Wulff-Burchfield EM, Mishra S, Farmakiotis D, Shyr Y, Warner JL, Choueiri TK, Cancer Consortium (2022) COVID-19 vaccination and breakthrough infections in patients with cancer. Ann Oncol 33:340–346. https://doi.org/10.1016/j.annonc.2021.12.006
Wang W, Kaelber DC, Xu R, Berger NA (2022) Breakthrough SARS-CoV-2 infections, hospitalizations, and mortality in vaccinated patients with cancer in the US between December 2020 and November 2021. JAMA Oncol. https://doi.org/10.1001/jamaoncol.2022.1096
Al Hajji Y, Taylor H, Starkey T, Lee LYW, Tilby M (2022) Antibody response to a third booster dose of SARS-CoV-2 vaccination in adults with haematological and solid cancer: a systematic review. Br J Cancer 127:1827–1836. https://doi.org/10.1038/s41416-022-01951-y
Zeng C, Evans JP, Chakravarthy K, Qu P, Reisinger S, Song NJ, Rubinstein MP, Shields PG, Li Z, Liu SL (2022) COVID-19 mRNA booster vaccines elicit strong protection against SARS-CoV-2 Omicron variant in patients with cancer. Cancer Cell 40:117–119. https://doi.org/10.1016/j.ccell.2021.12.014
Choueiri TK, Labaki C, Bakouny Z, Hsu CY, Schmidt AL, de Lima LG Jr, Hwang C, Singh SRK, Jani C, Weissmann LB, Griffiths EA, Halabi S, Wu U, Berg S, O’Connor TE, Wise-Draper TM, Panagiotou OA, Klein EJ, Joshi M, Yared F, Dutra MS, Gatson NTN, Blau S, Singh H, Nanchal R, McKay RR, Nonato TK, Quinn R, Rubinstein SM, Puc M, Mavromatis BH, Vikas P, Faller B, Zaren HA, Del Prete S, Russell K, Reuben DY, Accordino MK, Singh H, Friese CR, Mishra S, Rivera DR, Shyr Y, Farmakiotis D, Warner JL (2023) Breakthrough SARS-CoV-2 infections among patients with cancer following two and three doses of COVID-19 mRNA vaccines: a retrospective observational study from the COVID-19 and Cancer Consortium. Lancet Reg Health Am 19:100445. https://doi.org/10.1016/j.lana.2023.100445
Gong IY, Vijenthira A, Powis M, Calzavara A, Patrikar A, Sutradhar R, Hicks LK, Wilton D, Singh S, Krzyzanowska MK, Cheung MC (2023) Association of COVID-19 vaccination with breakthrough infections and complications in patients with cancer. JAMA Oncol 9:386–394. https://doi.org/10.1001/jamaoncol.2022.6815
Lee LYW, Ionescu MC, Starkey T, Little M, Tilby M, Tripathy AR, Mckenzie HS, Al-Hajji Y, Appanna N, Barnard M, Benny L, Burnett A, Cattell EL, Clark JJ, Khan S, Ghafoor Q, Panneerselvam H, Illsley G, Harper-Wynne C, Hattersley RJ, Lee AJ, Lomas O, Liu JK, McCauley A, Pang M, Pascoe JS, Platt JR, Patel G, Patel V, Potter VA, Randle A, Rigg AS, Robinson TM, Roques TW, Roux RL, Rozmanowski S, Taylor H, Tuthill MH, Watts I, Williams S, Beggs A, Iveson T, Lee SM, Middleton G, Middleton M, Protheroe A, Fittall MW, Fowler T, Johnson P, UK Coronavirus Cancer Programme (2022) COVID-19: third dose booster vaccine effectiveness against breakthrough coronavirus infection, hospitalisations and death in patients with cancer: a population-based study. Eur J Cancer 175:1–10. https://doi.org/10.1016/j.ejca.2022.06.038
Shapiro LC, Thakkar A, Campbell ST, Forest SK, Pradhan K, Gonzalez-Lugo JD, Quinn R, Bhagat TD, Choudhary GS, McCort M, Sica RA, Goldfinger M, Goel S, Anampa JD, Levitz D, Fromowitz A, Shah AP, Sklow C, Alfieri G, Racine A, Wolgast L, Greenberger L, Verma A, Halmos B (2022) Efficacy of booster doses in augmenting waning immune responses to COVID-19 vaccine in patients with cancer. Cancer Cell 40:3–5. https://doi.org/10.1016/j.ccell.2021.11.006
Malek AE, Cornejo PP, Daoud N, Alam M (2022) The mRNA COVID-19 vaccine in patients with cancer receiving checkpoint inhibitor therapy: what we know and what we don’t. Immunotherapy 14:91–94. https://doi.org/10.2217/imt-2021-0235
Waissengrin B, Agbarya A, Safadi E, Padova H, Wolf I (2021) Short-term safety of the BNT162b2 mRNA COVID-19 vaccine in patients with cancer treated with immune checkpoint inhibitors. Lancet Oncol 22:581–583. https://doi.org/10.1016/S1470-2045(21)00155-8
Luo B, Li J, Hou X, Yang Q, Zhou Y, Ye J, Wu X, Feng Y, Hu T, Xu Z, He Y, Sun J (2021) Indications for and contraindications of immune checkpoint inhibitors in cancer patients with COVID-19 vaccination. Future Oncol 17:3477–3484. https://doi.org/10.2217/fon-2021-0288
Trougakos IP, Terpos E, Alexopoulos H, Politou M, Paraskevis D, Scorilas A, Kastritis E, Andreakos E, Dimopoulos MA (2022) Adverse effects of COVID-19 mRNA vaccines: the spike hypothesis. Trends Mol Med 28:542–554. https://doi.org/10.1016/j.molmed.2022.04.007
Walle T, Bajaj S, Kraske JA, Rösner T, Cussigh CS, Kälber KA, Müller LJ, Strobel SB, Burghaus J, Kallenberger SM, Stein-Thöringer CK, Jenzer M, Schubert A, Kahle S, Williams A, Hoyler B, Zielske L, Skatula R, Sawall S, Leber MF, Kunes RZ, Krisam J, Fremd C, Schneeweiss A, Krauss J, Apostolidis L, Berger AK, Haag GM, Zschäbitz S, Halama N, Springfeld C, Kirsten R, Hassel JC, Jäger D, Investigators NCTANTICIPATE, Ungerechts G (2022) Cytokine release syndrome-like serum responses after COVID-19 vaccination are frequent and clinically inapparent under cancer immunotherapy. Nat Cancer. https://doi.org/10.1038/s43018-022-00398-7
Brest P, Mograbi B, Hofman P, Milano G (2022) COVID-19 vaccination and cancer immunotherapy: should they stick together? Br J Cancer 126:1–3. https://doi.org/10.1038/s41416-021-01618-0
Nelli F, Giannarelli D, Fabbri A, Silvestri MA, Berrios JRG, Virtuoso A, Marrucci E, Schirripa M, Mazzotta M, Onorato A, Panichi V, Topini G, Pessina G, Natoni F, Signorelli C, Chilelli MG, Primi F, Ruggeri EM (2022) Immunogenicity and early clinical outcome after two or three doses of SARS-CoV-2 mRNA-BNT162b2 vaccine in actively treated cancer patients: results from the prospective observational Vax-On-Third study. Ann Oncol 33:740–742. https://doi.org/10.1016/j.annonc.2022.04.002
AdviseDx SARS-CoV-2 IgG II. Package insert. Abbott laboratories; 2021. https://www.fda.gov/media/146371/download/. Accessed 18 May 2023
Saker K, Escuret V, Pitiot V, Massardier-Pilonchéry A, Paul S, Mokdad B, Langlois-Jacques C, Rabilloud M, Goncalves D, Fabien N, Guibert N, Fassier JB, Bal A, Trouillet-Assant S, Trabaud MA (2022) Evaluation of commercial anti-SARS-CoV-2 antibody assays and comparison of standardized titers in vaccinated health care workers. J Clin Microbiol 60:e0174621. https://doi.org/10.1128/JCM.01746-21
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247. https://doi.org/10.1016/j.ejca.2008.10.026
Holm S (1979) A simple sequentially rejective multiple test procedure. Scand J Stat 6:65–70
Garassino MC, Ribas A (2021) At the crossroads: COVID-19 and immune-checkpoint blockade for cancer. Cancer Immunol Res 9:261–264. https://doi.org/10.1158/2326-6066.CIR-21-0008
Shroff RT, Chalasani P, Wei R, Pennington D, Quirk G, Schoenle MV, Peyton KL, Uhrlaub JL, Ripperger TJ, Jergović M, Dalgai S, Wolf A, Whitmer R, Hammad H, Carrier A, Scott AJ, Nikolich-Žugich J, Worobey M, Sprissler R, Dake M, LaFleur BJ, Bhattacharya D (2021) Immune responses to two and three doses of the BNT162b2 mRNA vaccine in adults with solid tumors. Nat Med 7:2002–2011. https://doi.org/10.1038/s41591-021-01542-z
Oosting SF, van der Veldt AAM, Fehrmann RSN, GeurtsvanKessel CH, van Binnendijk RS, Dingemans AC, Smit EF, Hiltermann TJN, den Hartog G, Jalving M, Westphal TT, Bhattacharya A, de Wilt F, Boerma A, van Zijl L, Rimmelzwaan GF, Kvistborg P, van Els CACM, Rots NY, van Baarle D, Haanen JBAG, de Vries EGE (2022) Immunogenicity after second and third mRNA-1273 vaccination doses in patients receiving chemotherapy, immunotherapy, or both for solid tumours. Lancet Oncol S1470–2045(22):00203. https://doi.org/10.1016/S1470-2045(22)00203-0
Donahue RN, Marté JL, Goswami M, Toney NJ, Tsai YT, Gulley JL, Schlom J (2021) Interrogation of the cellular immunome of cancer patients with regard to the COVID-19 pandemic. J Immunother Cancer 9:e002087. https://doi.org/10.1136/jitc-2020-002087
Au L, Fendler A, Shepherd STC, Rzeniewicz K, Cerrone M, Byrne F, Carlyle E, Edmonds K, Del Rosario L, Shon J, Haynes WA, Ward B, Shum B, Gordon W, Gerard CL, Xie W, Joharatnam-Hogan N, Young K, Pickering L, Furness AJS, Larkin J, Harvey R, Kassiotis G, Gandhi S, Crick COVID-19 Consortium, Swanton C, Fribbens C, Wilkinson KA, Wilkinson RJ, Lau DK, Banerjee S, Starling N, Chau I, CAPTURE Consortium, Turajlic S (2021) Cytokine release syndrome in a patient with colorectal cancer after vaccination with BNT162b2. Nat Med 27:1362–1366. https://doi.org/10.1038/s41591-021-01387-6
Oosting SF, van der Veldt AAM, GeurtsvanKessel CH, Fehrmann RSN, van Binnendijk RS, Dingemans AC, Smit EF, Hiltermann TJN, den Hartog G, Jalving M, Westphal TT, Bhattacharya A, van der Heiden M, Rimmelzwaan GF, Kvistborg P, Blank CU, Koopmans MPG, Huckriede ALW, van Els CACM, Rots NY, van Baarle D, Haanen JBAG, de Vries EGE (2021) mRNA-1273 COVID-19 vaccination in patients receiving chemotherapy, immunotherapy, or chemoimmunotherapy for solid tumours: a prospective, multicentre, non-inferiority trial. Lancet Oncol 22:1681–1691. https://doi.org/10.1016/S1470-2045(21)00574-X
Song P, Zhang D, Cui X, Zhang L (2020) Meta-analysis of immune-related adverse events of immune checkpoint inhibitor therapy in cancer patients. Thorac Cancer 11:2406–2430. https://doi.org/10.1111/1759-7714.13541
Chen YW, Tucker MD, Beckermann KE, Iams WT, Rini BI, Johnson DB (2021) COVID-19 mRNA vaccines and immune-related adverse events in cancer patients treated with immune checkpoint inhibitors. Eur J Cancer 155:291–293. https://doi.org/10.1016/j.ejca.2021.07.017
Strobel SB, Machiraju D, Kälber KA, Hassel JC (2021) Immune-related adverse events of COVID-19 vaccination in skin cancer patients receiving immune-checkpoint inhibitor treatment. Cancer Immunol Immunother. https://doi.org/10.1007/s00262-021-03133-w
Hibino M, Uryu K, Takeda T, Kunimatsu Y, Shiotsu S, Uchino J, Hirai S, Yamada T, Okada A, Hasegawa Y, Hiranuma O, Chihara Y, Kamada R, Tobe S, Maeda K, Horiuchi S, Kondo T, Takayama K (2022) Safety and immunogenicity of mRNA vaccines against severe acute respiratory syndrome coronavirus 2 in patients with lung cancer receiving immune checkpoint inhibitors: a multicenter observational study in Japan. J Thorac Oncol S1556–0864(22):00295–00297. https://doi.org/10.1016/j.jtho.2022.05.015
Gilbert D, Hu J, Medina T, Kessler ER, Lam ET (2023) Safety of COVID-19 vaccines in subjects with solid tumor cancers receiving immune checkpoint inhibitors. Hum Vaccin Immunother 9:2207438. https://doi.org/10.1080/21645515.2023.2207438
Widman AJ, Cohen B, Park V, McClure T, Wolchok J, Kamboj M (2022) Immune-related adverse events among COVID-19-Vaccinated patients with cancer receiving immune checkpoint blockade. J Natl Compr Canc Netw 20:1134–1138. https://doi.org/10.6004/jnccn.2022.7048
Vahabi M, Ghazanfari T, Sepehrnia S (2022) Molecular mimicry, hyperactive immune system, and SARS-COV-2 are three prerequisites of the autoimmune disease triangle following COVID-19 infection. Int Immunopharmacol 112:109183. https://doi.org/10.1016/j.intimp.2022.109183
Pezzaioli LC, Gatta E, Bambini F, Facondo P, Gava M, Cavadini M, Buoso C, Di Lodovico E, Rotondi M, Ferlin A, Cappelli C (2022) Endocrine system after 2 years of COVID-19 vaccines: a narrative review of the literature. Front Endocrinol (Lausanne) 13:1027047. https://doi.org/10.3389/fendo.2022.1027047
Wong CKH, Lui DTW, Xiong X, Chui CSL, Lai FTT, Li X, Wan EYF, Cheung CL, Lee CH, Woo YC, Au ICH, Chung MSH, Cheng FWT, Tan KCB, Wong ICK (2022) Risk of thyroid dysfunction associated with mRNA and inactivated COVID-19 vaccines: a population-based study of 2.3 million vaccine recipients. BMC Med 20:339. https://doi.org/10.1186/s12916-022-02548-1
Wright JJ, Powers AC, Johnson DB (2021) Endocrine toxicities of immune checkpoint inhibitors. Nat Rev Endocrinol 17:389–399. https://doi.org/10.1038/s41574-021-00484-3
Heine A, Juranek S, Brossart P (2021) Clinical and immunological effects of mRNA vaccines in malignant diseases. Mol Cancer 20:52. https://doi.org/10.1186/s12943-021-01339-1
Denis M, Duruisseaux M, Brevet M, Dumontet C (2020) How can immune checkpoint inhibitors cause hyperprogression in solid tumors? Front Immunol 11:492. https://doi.org/10.3389/fimmu.2020.00492
Bersanelli M, Giannarelli D, Castrignanò P, Fornarini G, Panni S, Mazzoni F, Tiseo M, Rossetti S, Gambale E, Rossi E, Papa A, Cortellini A, Lolli C, Ratta R, Michiara M, Milella M, De Luca E, Sorarù M, Mucciarini C, Atzori F, Banna GL, La Torre L, Vitale MG, Massari F, Rebuzzi SE, Facchini G, Schinzari G, Tomao S, Bui S, Vaccaro V, Procopio G, De Giorgi U, Santoni M, Ficorella C, Sabbatini R, Maestri A, Natoli C, De Tursi M, Di Maio M, Rapacchi E, Pireddu A, Sava T, Lipari H, Comito F, Verzoni E, Leonardi F, Buti S (2018) Influenza vaccine indication during therapy with immune checkpoint inhibitors: a transversal challenge. INVIDIa Study Immunother 10:1229–1239. https://doi.org/10.2217/imt-2018-0080
Valachis A, Rosén C, Koliadi A, Digkas E, Gustavsson A, Nearchou A, Ullenhag GJ (2021) Improved survival without increased toxicity with influenza vaccination in cancer patients treated with checkpoint inhibitors. Oncoimmunology 10:1886725. https://doi.org/10.1080/2162402X.2021.1886725
Chong CR, Park VJ, Cohen B, Postow MA, Wolchok JD, Kamboj M (2020) Safety of inactivated influenza vaccine in cancer patients receiving immune checkpoint inhibitors. Clin Infect Dis 70:193–199. https://doi.org/10.1093/cid/ciz202
Mei Q, Hu G, Yang Y, Liu B, Yin J, Li M, Huang Q, Tang X, Böhner A, Bryant A, Kurts C, Yuan X, Li J (2022) Impact of COVID-19 vaccination on the use of PD-1 inhibitor in treating patients with cancer: a real-world study. J Immunother Cancer 10:e004157. https://doi.org/10.1136/jitc-2021-004157
Gleiss A, Oberbauer R, Heinze G (2018) An unjustified benefit: immortal time bias in the analysis of time-dependent events. Transpl Int 31:125–130. https://doi.org/10.1111/tri.13081
Kim HK, Heo MH, Lee HS, Sun JM, Lee SH, Ahn JS, Park K, Ahn MJ (2017) Comparison of RECIST to immune-related response criteria in patients with non-small cell lung cancer treated with immune-checkpoint inhibitors. Cancer Chemother Pharmacol 80:591–598. https://doi.org/10.1007/s00280-017-3396-4
Sullivan RJ, Weber JS (2022) Immune-related toxicities of checkpoint inhibitors: mechanisms and mitigation strategies. Nat Rev Drug Discov 21:495–508. https://doi.org/10.1038/s41573-021-00259-5
Gauci ML, Coutzac C, Houot R, Marabelle A, Lebbé C, FITC (2021) SARS-CoV-2 vaccines for cancer patients treated with immunotherapies: recommendations from the French society for immunotherapy of cancer (FITC). Eur J Cancer 148:121–123. https://doi.org/10.1016/j.ejca.2021.02.003
Funding
This research did not receive any specific grant from agencies in the public, commercial, or not-for-profit sectors and no sources of funding were used to assist in the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
FN and EMR designed the study and wrote the manuscript. DG performed the statistical analysis. AF, JRGB, AV, EM, CF, MS, CS, MGC, and FP enrolled the patients, collected clinical and laboratory data and setup the database. VP, GT, and MAS performed serological testing, and contributed to the interpretation of the data. All authors discussed the results and critically revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
This study was approved by the referring Ethics Committee (Comitato Etico Lazio1, Rome, Italy; protocol number: 1407/CE Lazio1). All patients were required to provide written informed consent before participating in this study. All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nelli, F., Giannarelli, D., Fabbri, A. et al. Immune-related adverse events and disease outcomes after the third dose of SARS-CoV-2 mRNA-BNT162b2 vaccine in cancer patients receiving immune checkpoint inhibitors. Cancer Immunol Immunother 72, 3217–3228 (2023). https://doi.org/10.1007/s00262-023-03489-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-023-03489-1