Abstract
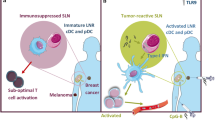
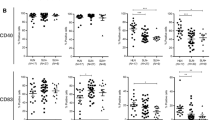
Triple negative breast cancer (TNBC) is a significant clinical problem to which immunotherapeutic strategies have been applied with limited success. Using the syngeneic E0771 TNBC mouse model, this work explores the potential for antitumor CD8+ T cell immunity to be primed extratumorally in lymphoid tissues and therapeutically leveraged. CD8+ T cell viability and responses within the tumor microenvironment (TME) were found to be severely impaired, effects coincident with local immunosuppression that is recapitulated in lymphoid tissues in late stage disease. Prior to onset of a locally suppressed immune microenvironment, however, CD8+ T cell priming within lymph nodes (LN) that depended on tumor lymphatic drainage remained intact. These results demonstrate tumor-draining LNs (TdLN) to be lymphoid tissue niches that support the survival and antigenic priming of CD8+ T lymphocytes against lymph-draining antigen. The therapeutic effects of and CD8+ T cells response to immune checkpoint blockade were furthermore improved when directed to LNs within the tumor-draining lymphatic basin. Therefore, TdLNs represent a unique potential tumor immunity reservoir in TNBC for which strategies may be developed to improve the effects of ICB immunotherapy.








Similar content being viewed by others
Code availability
Not applicable.
References
Siegel RL, Miller KD, Jemal A (2019) Cancer statistics, 2019. CA Cancer J Clin 69:7–34. https://doi.org/10.3322/caac.21551
Ferlay J, Soerjomataram I, Dikshit R et al (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136:E359–E386. https://doi.org/10.1002/ijc.29210
Foulkes WD, Smith IE, Reis-Filho JS (2010) Triple-negative breast cancer. N Engl J Med 363:1938–1948
Seidel JA, Otsuka A, Kabashima K (2018) Anti-PD-1 and anti-CTLA-4 therapies in cancer: mechanisms of action, efficacy, and limitations. Front Oncol 8:86. https://doi.org/10.3389/fonc.2018.00086
Brahmer JR, Tykodi SS, Chow LQM et al (2012) Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 366:2455–2465. https://doi.org/10.1056/NEJMoa1200694
Schmid P, Adams S, Rugo HS et al (2018) Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med 379:2108–2121. https://doi.org/10.1056/NEJMoa1809615
Wang T, Wang C, Wu J et al (2017) The different T-cell receptor repertoires in breast cancer tumors, draining lymph nodes, and adjacent tissues. Cancer Immunol Res 5:148–157. https://doi.org/10.1158/2326-6066.CIR-16-0107
Crosby EJ, Wei J, Yang XY et al (2017) Complimentary mechanisms of dual checkpoint blockade expand unique T-cell repertoires and activate adaptive anti-tumor immunity in triple-negative breast tumors. Oncoimmunology 7:e1421891. https://doi.org/10.1080/2162402x.2017.1421891
Shiota T, Miyasato Y, Ohnishi K et al (2016) The clinical significance of CD169-positive lymph node macrophage in patients with breast cancer. PLoS ONE 11:1–13. https://doi.org/10.1371/journal.pone.0166680
O’Melia MJ, Lund AW, Thomas SN (2019) The biophysics of lymphatic transport: engineering tools and immunological consequences. Science 22:28–43. https://doi.org/10.1016/j.isci.2019.11.005
Murphy K, Weaver C (2017) Janeway’s Immunobiology, 9th edn. Taylor & Francis, New York
Wiig H, Swartz MA (2012) Interstitial fluid and lymph formation and transport: physiological regulation and roles in inflammation and cancer. Physiol Rev 92:1005–1060. https://doi.org/10.1152/physrev.00037.2011
Huxley VH, Scallan J (2011) Lymphatic fluid: exchange mechanisms and regulation. J Physiol 589:2935–2943. https://doi.org/10.1113/jphysiol.2011.208298
Zawieja DC (2009) Contractile physiology of lymphatics. Lymphat Res Biol 7:87–96. https://doi.org/10.1089/lrb.2009.0007
Reddy ST, Van Der Vlies AJ, Simeoni E et al (2007) Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat Biotechnol 25:1159–1164. https://doi.org/10.1038/nbt1332
Rohner NA, Thomas SN (2016) Melanoma growth effects on molecular clearance from tumors and biodistribution into systemic tissues versus draining lymph nodes. J Control Release 223:99–108. https://doi.org/10.1016/j.jconrel.2015.12.027
Rohner NA, Thomas SN (2017) Flexible macromolecule versus rigid particle retention in the injected skin and accumulation in draining lymph nodes are differentially influenced by hydrodynamic size. ACS Biomater Sci Eng 3:153–159. https://doi.org/10.1021/acsbiomaterials.6b00438
Allan RS, Waithman J, Bedoui S et al (2006) Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity 25:153–162. https://doi.org/10.1016/j.immuni.2006.04.017
Thomas SN, Rutkowski JM, Pasquier M et al (2012) Impaired humoral immunity and tolerance in K14-VEGFR-3-Ig mice that lack dermal lymphatic drainage. J Immunol 189:2181–2190. https://doi.org/10.4049/jimmunol.1103545
Loo CP, Nelson NA, Lane RS et al (2017) Lymphatic vessels balance viral dissemination and immune activation following cutaneous viral infection. Cell Rep 20:3176–3187. https://doi.org/10.1016/j.celrep.2017.09.006
Platt AM, Kutkowski JM, Martel C et al (2013) Normal dendritic cell mobilization to lymph nodes under conditions of severe lymphatic hypoplasia. J Immunol 190:4608–4620. https://doi.org/10.4049/jimmunol.1202600.Normal
Nakamura R, Sakakibara M, Nagashima T et al (2009) Accumulation of regulatory T cells in sentinel lymph nodes is a prognostic predictor in patients with node-negative breast cancer. Eur J Cancer 45:2123–2131. https://doi.org/10.1016/j.ejca.2009.03.024
Liyanage UK, Moore TT, Joo H-G et al (2002) Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol 169:2756–2761. https://doi.org/10.4049/jimmunol.169.5.2756
Danilin S, Merkel AR, Johnson JR et al (2012) Myeloid-derived suppressor cells expand during breast cancer progression and promote tumor-induced bone destruction. Oncoimmunology 1:1484–1494
van Pul KM, Vuylsteke RJCLM, van de Ven R et al (2020) Selectively hampered activation of lymph node-resident dendritic cells precedes profound T cell suppression and metastatic spread in the breast cancer sentinel lymph node. J Immunother Cancer 7:133. https://doi.org/10.1186/s40425-019-0605-1
Foulds GA, Vadakekolathu J, Abdel-Fatah TMA et al (2018) Immune-phenotyping and transcriptomic profiling of peripheral blood mononuclear cells from patients with breast cancer: identification of a 3 gene signature which predicts relapse of triple negative breast cancer. Front Immunol 9:2028. https://doi.org/10.3389/fimmu.2018.02028
Satthaporn S, Robins A, Vassanasiri W et al (2004) Dendritic cells are dysfunctional in patients with operable breast cancer. Cancer Immunol Immunother 53:510–518. https://doi.org/10.1007/s00262-003-0485-5
Gil Del Alcazar CR, Huh SJ, Ekram MB et al (2017) Immune escape in breast cancer during in situ to invasive carcinoma transition. Cancer Discov 7:1098–1115. https://doi.org/10.1158/2159-8290.CD-17-0222
Mahmoud SMA, Paish EC, Powe DG et al (2011) Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol 29:1949–1955. https://doi.org/10.1200/JCO.2010.30.5037
Savas P, Virassamy B, Ye C et al (2018) Single-cell profiling of breast cancer T cells reveals a tissue-resident memory subset associated with improved prognosis. Nat Med 24:986–993. https://doi.org/10.1038/s41591-018-0078-7
Guo L, Cao C, Goswami S et al (2020) Tumoral PD-1hiCD8+ T cells are partially exhausted and predict favorable outcome in triple-negative breast cancer. Clin Sci 134:711–726. https://doi.org/10.1042/CS20191261
Chang AY, Bhattacharya N, Mu J et al (2013) Spatial organization of dendritic cells within tumor draining lymph nodes impacts clinical outcome in breast cancer patients. J Transl Med 11:1–12
Duvall CL, Taylor WR, Weiss D, Guldberg RE (2004) Quantitative microcomputed tomography analysis of collateral vessel development after ischemic injury. Am J Physiol Hear Circ Physiol 287:H302–H310. https://doi.org/10.1152/ajpheart.00928.2003
Li X, Li M, Lian Z et al (2016) Prognostic role of programmed death ligand-1 expression in breast cancer: a systematic review and meta-analysis. Target Oncol 11:753–761. https://doi.org/10.1007/s11523-016-0451-8
Kim IS, Gao Y, Welte T et al (2019) Immuno-subtyping of breast cancer reveals distinct myeloid cell profiles and immunotherapy resistance mechanisms. Nat Cell Biol 21:1113–1126. https://doi.org/10.1038/s41556-019-0373-7
Green DR, Droin N, Pinkoski M (2003) Activation-induced cell death in T cells. Immunol Rev 193:70–81. https://doi.org/10.1034/j.1600-065X.2003.00051.x
Kabelitz D, Pohl T, Pechhold K (1993) Activation-induced cell death (apoptosis) of mature peripheral T lymphocytes. Trends Immunol 14:338–339
Ucker DS, Hebshi LD, Blomquist JE, Torbett BE (1994) Physiological T-cell death: susceptibility is modulated by activation, aging, and transformation, but the mechanism is constant. Immunol Rev 142:273–299. https://doi.org/10.1111/j.1600-065X.1994.tb00893.x
Hanahan D, Weinberg RA (2000) The Hallmarks of Cancer. Cell 100:57–70. https://doi.org/10.1007/s00262-010-0968-0
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674. https://doi.org/10.1016/j.cell.2011.02.013
Shibuya M (2011) Vascular endothelial growth factor (VEGF) and its receptor (VEGFR) signaling in angiogenesis: a crucial target for anti- and pro-angiogenic therapies. Genes Cancer 2:1097–1105. https://doi.org/10.1177/1947601911423031
Carmeliet P (2005) VEGF as a key mediator of angiogenesis in cancer. Oncology 69:4–10. https://doi.org/10.1159/000088478
Nagy JA, Dvorak AM, Dvorak HF (2012) Vascular hyperpermeability, angiogenesis, and stroma generation. Cold Spring Harb Perspect Med 2:1–14. https://doi.org/10.1101/cshperspect.a006544
Nagy JA, Feng D, Vasile E et al (2006) Permeability properties of tumor surrogate blood vessels induced by VEGF-A. Lab Investig 86:767–780. https://doi.org/10.1038/labinvest.3700436
Schneider BP, Gray RJ, Radovich M et al (2013) Prognostic and predictive value of tumor vascular endothelial growth factor gene amplification in metastatic breast cancer treated with paclitaxel with and without bevacizumab; results from ECOG 2100 trial. Clin Cancer Res 19:1281–1289. https://doi.org/10.1158/1078-0432.CCR-12-3029
Su J-C, Mar A-C, Wu S-H et al (2016) Disrupting VEGF-A paracrine and autocrine loops by targeting SHP-1 suppresses triple negative breast cancer metastasis. Sci Rep 6:28888. https://doi.org/10.1038/srep28888
Bahnassy A, Mohanad M, Ismail MF et al (2015) Molecular biomarkers for prediction of response to treatment and survival in triple negative breast cancer patients from Egypt. Exp Mol Pathol 99:303–311. https://doi.org/10.1016/j.yexmp.2015.07.014
Sweat RS, Sloas DC, Murfee WL (2014) VEGF-C induces lymphangiogenesis and angiogenesis in the rat mesentery culture model. Microcirculation 21:532–540. https://doi.org/10.1111/micc.12132
Vaahtomeri K, Karaman S, Mäkinen T, Alitalo K (2017) Lymphangiogenesis guidance by paracrine and pericellular factors. Genes Dev 31:1615–1634. https://doi.org/10.1101/gad.303776.117
Lund AW, Wagner M, Fankhauser M et al (2016) Lymphatic vessels regulate immune microenvironments in human and murine melanoma. J Clin Investig 126:3389–3402. https://doi.org/10.1172/JCI79434
Stacker SA, Williams SP, Karnezis T et al (2014) Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nat Rev Cancer 14:159–172. https://doi.org/10.1038/nrc3677
Kitano M, Yamazaki C, Takumi A et al (2016) Imaging of the cross-presenting dendritic cell subsets in the skin-draining lymph node. Proc Natl Acad Sci 113:1044–1049. https://doi.org/10.1073/pnas.1513607113
Pucci F, Garris C, Lai CP et al (2016) SCS macrophages suppress melanoma by restricting tumor-derived vesicle-B cell interactions. Science (80-) 352:242–246. https://doi.org/10.1126/science.aaf1328
Hood JL, San Roman S, Wickline SA (2011) Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis. Cancer Res 71:3792–3801. https://doi.org/10.1158/0008-5472.CAN-10-4455
Broggi MAS, Maillat L, Clement CC et al (2019) Tumor-associated factors are enriched in lymphatic exudate compared to plasma in metastatic melanoma patients. J Exp Med 216:1091–1107. https://doi.org/10.1084/jem.20181618
Spranger S, Spaapen RM, Zha Y et al (2013) Up-regulation of PD-L1, IDO, and tregs in the melanoma tumor microenvironment is driven by CD8+ T Cells. Sci Transl Med. https://doi.org/10.1126/scitranslmed.3006504
Platt AM, Randolph GJ (2013) Dendritic cell migration through the lymphatic vasculature to lymph nodes, 1st edn. Elsevier Inc., Amsterdam
Schineis P, Runge P, Halin C (2019) Cellular traffic through afferent lymphatic vessels. Vasc Pharmacol 112:31–41. https://doi.org/10.1016/j.vph.2018.08.001
Harrell MI, Iritani BM, Ruddell A (2008) Lymph node mapping in the mouse. J Immunol Methods 332:170–174. https://doi.org/10.1016/j.jim.2007.11.012
Im SJ, Hashimoto M, Gerner MY et al (2016) Defining CD8+T cells that provide the proliferative burst after PD-1 therapy. Nature 537:417–421. https://doi.org/10.1038/nature19330
Miller BC, Sen DR, Al Abosy R et al (2019) Subsets of exhausted CD8+ T cells differentially mediate tumor control and respond to checkpoint blockade. Nat Immunol 20:326–336. https://doi.org/10.1038/s41590-019-0312-6
Gupta PK, Godec J, Wolski D et al (2015) CD39 expression identifies terminally exhausted CD8+ T cells. PLoS Pathog 11:1–21. https://doi.org/10.1371/journal.ppat.1005177
Francis DM, Manspeaker MP, Schudel A et al (2020) Blockade of immune checkpoints in lymph nodes through locoregional delivery augments cancer immunotherapy. Sci Transl Med 12:eaay3575
Jansen CS, Prokhnevska N, Master VA et al (2019) An intra-tumoral niche maintains and differentiates stem-like CD8 T cells. Nature 576:465–470. https://doi.org/10.1038/s41586-019-1836-5
Cabrita R, Lauss M, Sanna A et al (2020) Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature 577:561–565. https://doi.org/10.1038/s41586-019-1914-8
Petitprez F, de Reyniès A, Keung EZ et al (2020) B cells are associated with survival and immunotherapy response in sarcoma. Nature 577:556–560. https://doi.org/10.1038/s41586-019-1906-8
Horton BL, Williams JB, Cabanov A et al (2018) Intratumoral CD8+ T-cell apoptosis is a major component of T-cell dysfunction and impedes antitumor immunity. Cancer Immunol Res 6:14–24. https://doi.org/10.1158/2326-6066.CIR-17-0249
Cursiefen C, Chen L, Borges LP et al (2004) VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J Clin Investig 113:1040–1050. https://doi.org/10.1172/JCI200420465.1040
Hirakawa S, Kodama S, Kunstfeld R et al (2005) VEGF-A induces tumor and sentinel lymph node lymphangiogenesis and promotes lymphatic metastasis. J Exp Med 201:1089–1099. https://doi.org/10.1084/jem.20041896
Halin C, Tobler NE, Vigl B et al (2007) VEGF-A produced by chronically inflamed tissue induces lymphangiogenesis in draining lymph nodes. Blood 110:3158–3167. https://doi.org/10.1182/blood-2007-01-066811
Rahma OE, Hodi FS (2019) The intersection between tumor angiogenesis and immune suppression. Clin Cancer Res 25:5449–5457. https://doi.org/10.1158/1078-0432.CCR-18-1543
Naik AM, Fey J, Gemignani M et al (2004) The risk of axillary relapse after sentinel lymph node biopsy for breast cancer is comparable with that of axillary lymph node dissection. Ann Surg 240:462–471. https://doi.org/10.1097/01.sla.0000137130.23530.19
Pesce C, Morrow M (2013) The need for lymph node dissection in nonmetastatic breast cancer. Annu Rev Med 64:119–129. https://doi.org/10.1146/annurev-med-052511-135500
Giuliano AE, Hunt KK, Ballman KV et al (2011) Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis. J Am Med Assoc 305:569–575. https://doi.org/10.1001/jama.2011.90
Acknowledgements
We thank Paul Archer for technical assistance.
Funding
This work was supported by a Komen Foundation Career Catalyst Grant CCR15330478, US National Institutes of Health Grants R01CA207619 (SNT), U01CA214354 (SNT), T32GM008433 (MJO), S10OD016264, and Georgia CORE/It’s the Journey. MPM was a National Science Foundation Graduate Research Fellow.
Author information
Authors and Affiliations
Contributions
MJO and SNT designed all experiments and wrote manuscript. MJO carried out all experiments and analyzed all data. MPM assisted in carrying out therapeutic experiments. All authors reviewed and approved the manuscript for submission.
Corresponding author
Ethics declarations
Conflict of interest
Not applicable.
Ethics approval
All animal work was approved by the Georgia Institute of Technology Institutional Animal Care and Use Committee.
Consent to participate
Not applicable.
Consent for publication
All authors have reviewed the manuscript and consent to publication.
Availability of data and material
Data and materials described here will be made available upon request.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
O’Melia, M.J., Manspeaker, M.P. & Thomas, S.N. Tumor-draining lymph nodes are survival niches that support T cell priming against lymphatic transported tumor antigen and effects of immune checkpoint blockade in TNBC. Cancer Immunol Immunother 70, 2179–2195 (2021). https://doi.org/10.1007/s00262-020-02792-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-020-02792-5