Abstract
Introduction
Natural killer cells (NK) are often believed to play a positive role in the antitumor immune response. However, this is not the case for patients with advanced pancreatic cancer. This study was performed to determine the unique subtype of “educated” NK cells and their prognostic value in patients with advanced pancreatic cancer.
Methods
We divided 378 eligible patients into a derivation cohort (September 2010 to December 2014, n = 239) and a validation cohort (January 2015 to April 2016, n = 139). Flow cytometry was performed to analyze NK cells. Enzyme-linked immunosorbent assay was used to detect interleukin-2 (IL-2), interferon gamma (IFN-γ) and tumor necrosis factor alpha (TNF-α) production. The Kaplan–Meier method and the Cox proportional hazards model were used.
Results
Survival analysis showed that a high density of NK cells accompanied by a high neutrophil-to-lymphocyte ratio was associated with reduced overall survival in both the derivation and validation cohorts. Multivariable analysis also showed that high NK infiltration (HR 1.45, 95% CI 1.17 to 1.79, p = 0.001) was an independent prognostic factor. In these patients, high NK infiltration was associated with reduced levels of IL-2, IFN-γ and TNF-α, although only IFN-γ reached statistical significance, which accounted for this unique phenomenon.
Discussion
Natural killer cells in patients with advanced pancreatic cancer are a unique subtype with anergic features. A high density of NKs predicts poor survival in these patients, possibly because an active inflammatory response and reduced secretion of IL-2, IFN-γ and TNF-α inhibit NK activation.
Similar content being viewed by others
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is the fourth leading cause of cancer-related death in the United States and Europe [2]. A unique microenvironment with abundant stroma is a hallmark of PDAC that distinguishes it from other tumors. Although the role of the tumor microenvironment in PDAC has been extensively studied, interactions of specific stromal components and tumor cells are not well understood.
It is widely acknowledged that natural killer cells (NK cells) play a protective role in spontaneous antitumor response. By definition, NK cells are a subset of immunocytes that kill target cells “naturally”, displaying cytotoxic functions and cytokine secretion. Lacking expression of cluster of differentiation-3 (CD3), the neural cell adhesion molecule (NCAM), also known as CD56, is usually considered a marker of NK cells and is found on a subset of T cells. In contrast to peripheral blood NK cells, studies of tumor-infiltrating NK cells are limited. Unexpectedly, our previous work found increased levels of NK cells in resectable or borderline-resectable PDAC patients compared to the normal population [3]. Similar results were also found in non-small-cell lung cancer (NSCLC) [4]. Unlike normal immune cells, NK cells found in PDAC tended to be a unique subtype of immune cell “educated” by tumor cells. Thus, we aimed to understand how these “educated” intratumoral NK cells function in PDAC.
Normally, activation of NK cells requires multiple signals, including interleukin-2 (IL-2), IL-12, IL-15, and IL-18. Endogenous T cell-derived IL-2 modulates NK cell functions by multiple mechanisms [5]. In the presence of IL-2, NK cells are activated following the release interferon-γ (IFN-γ). NK cells release cytokines and chemokines when activated, including interferon gamma (IFN-γ) and tumor necrosis factor alpha (TNF-α) [6].
The neutrophil-to-lymphocyte ratio (NLR) is defined as the ratio between the absolute number of neutrophils and lymphocytes. Previous studies have shown that a high NLR value is associated with a poor prognosis in PDAC patients treated with surgery [7, 8] or chemotherapy [9]. We analyzed blood neutrophils and lymphocytes and calculated the NLR to verify our hypothesis.
In this study, the percentage of CD3−CD16+CD56+ NK cells was examined in a large cohort. In addition, several other biomarkers of PDAC were also analyzed. Enzyme-linked Immunosorbent Assay (ELISA) was performed to measure IL-2. The relationship between the NK cell number and the prognosis of advanced PDAC patients is discussed.
Materials and methods
Design and patients
The study was approved by the Ethics Committee of Fudan University Shanghai Cancer Center (FDUSCC) and met ethical guidelines of the World Medical Association Declaration of Helsinki. Written informed consent was acquired at FDUSCC.
Eligible patients who met the following standards were enrolled in the study. Pancreatic ductal adenocarcinomas confirmed by pathology were selected for analysis; stage III and IV tumors were included as defined by the 7th edition of the American Joint Committee on Cancer (Chicago, IL, USA) classification. Patients were required to have adequate renal and liver functions to tolerate the side effects of chemotherapy. If icterus appeared, appropriate treatment was performed to relieve the jaundice. No other major cancers were detected. Initial medical care was conducted at FUDSCC.
Exclusion criteria of this study were as follows: patients with stage I and II PDAC; intolerance to chemotherapy; presence of other tumors; incomplete medical records; and presence of active infections or immune disease prior to receiving major medical care.
The patients were divided into derivation (September 2010–December 2014, n = 239) and validation (January 2015–April 2016, n = 139) cohorts. The primary endpoint was overall survival (OS). OS was defined as the date of diagnosis to the date of death or last follow-up. An independent analyzer conducted the follow-up procedure and calculated the OS. June 1, 2017, was the date of last follow-up.
Blood samples and flow cytometry
Blood samples were collected before receiving medical interferon. NK cells were analyzed in blood but not other tissues. Detailed procedures of flow cytometry using BD Multitest 6-color TBNK reagent (BD Pharmingen, USA) have been described previously. In brief, blood cells were treated with red blood cell lysis buffer (Beyotime, China) and then labeled with fluorochrome-conjugated monoclonal antibodies and incubated at 4 °C for 20 min. The antibodies used were anti-CD56 (clone MEM-188), anti-CD16, and anti-CD3 (eBioscience). CD3−CD16+CD56+ was considered the marker of NK cells.
Enzyme-linked immunosorbent assay and clinical data
Human IL-2, IFN-γ, and TNF-α Quantikine ELISA Kits were purchased from R&D Systems (USA). The ELISA was conducted according to the manufacturer’s instructions. The standard concentration was plotted on the horizontal axis, while the optical density (OD) value at 450 nm was plotted on the vertical axis. Neutrophil and lymphocyte numbers at least 3 days before major primary treatment were obtained from routine clinical data from the Hospital Information System (HIS) of FDUSCC.
Statistical analyses
The Kaplan–Meier method was used to estimate overall survival. Log-rank tests were performed to compare the survival distributions of the two samples. The hazard ratio was estimated with a Cox proportional hazards model. The Wilcoxon rank test and χ2 test were used where appropriate. GraphPad Prism 7.0 (GraphPad Software, Inc., USA) software was used to create the artwork. The data are shown as the means ± SE. Statistical analyses were conducted using STATA 13.0 (StataCorp LP, College Station, TX, USA). A p value < 0.05 was considered significant.
Results
Patient characteristics
In total, 446 patients with advanced PDAC were initially included in the analysis. The cohort was divided into derivation (September 2010–December 2014) and validation (January 2015–April 2016) cohorts. The study participant flow diagram is presented in Fig. 1. Among these patients, 24 in the derivation cohort and 9 in the validation cohort did not have complete clinical data (total = 33, 7.40%). Furthermore, 21 and 14 patients in the derivation and validation cohorts were lost to follow-up, respectively. The lost-to-follow-up rate was 7.85%. All participants lost to follow-up or with incomplete clinical data were removed from the study, and 378 patients were ultimately included in the final analysis. Among them, 239 patients were grouped into the derivation cohort, and 139 patients were grouped into the validation cohort. Characteristics of both cohorts are listed on Table 1. There were no obvious differences in baseline characteristics between the derivation and validation cohorts, and median OS was 5 ± 0.18 [95% confidence interval (CI) 5–5] and 5 ± 0.29 (95% CI 5–5), respectively.
A cut-off value of 20% NKs among blood cells was selected as the median value for all participants. A high NLR value was defined as NLR equal to or greater than 3.1, as described in our previous study [9].
High NK cell density indicates a poor prognosis and is accompanied by high NLR
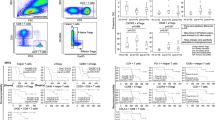
Survival curves for patients stratified across a specific NK cell cut-off value are presented in Fig. 2. Unexpectedly, we found that a high density of NK cells indicated shorter OS in the derivation cohort (log-rank test, p value = 0.0205). The median OS for the high NK group was 4 ± 0.32 months (95% CI 4–5), whereas the low NK group showed a median OS of 6 ± 0.30 months (95% CI 5–6). These results were confirmed in the validation group (p = 0.0145).
These results represent a completely different paradigm from our previous understanding of NK cells. Furthermore, we chose NLR, a commonly used laboratory marker obtained from complete blood counts (CBC), to test the efficacy of the group. In this cohort, the data indicate that high NLR was associated with worse outcomes (Supplement Figure 1), and a high number of circulating NK cells with a high NLR value was correlated with poor prognosis in both the derivation and validation cohorts.
Univariate and multivariate analyses by the Cox proportional hazards model are shown in Table 2. Multivariate analyses found that age (hazard ratio, HR 1.34, 95% confidence interval (95% CI) 1.08–1.67, p = 0.008), absence of metastasis (HR 0.72, 95% CI 0.57–0.90, p = 0.005), NLR (HR 1.48, 95% CI 1.19–1.83, p < 0.001), and NK cells (HR 1.45, 95% CI 1.17–1.79, p = 0.001) were independent prognostic factors.
Reduced levels of IL-2, IFN-γ, and TNF-α are associated with NK anergy
The abnormal NK cell distribution in the population aroused our interest, as the distribution of NLR values was normal in the cohorts. We hypothesized that the NK cells we measured were different from traditional NK cells. We speculated that they could be a unique subtype with impaired functions, which may be associated with NLR. Activation of NK cells requires stimulation from IL-2, and NLR has been reported to serve as a biomarker for the response to IL-2 in renal cell carcinoma [10]. Activated NK cells release IFN-γ and TNF-α as well. Therefore, half of the patients from the validation cohort were randomly assigned to undergo ELISA to measure serum IL-2, IFN-γ and TNF-α. A randomization sequence was created using STATA 13.0 software. A total of 62 blood samples were collected. Five cases were excluded from the ELISA due to a lack of adequate sample volume, and 57 samples were analyzed. Groups were divided into a low NK group and a high NK group. Interestingly, as shown in Fig. 3, the mean values of IL-2, IFN-γ and TNF-α were lower in the high NK group, although only IFN-γ showed statistical significance (low NK group, n = 26; high NK group, n = 31; IL-2, p = 0.0780; TNF-α, p = 0.0971; IFN-γ, p = 0.0425).
Discussion
Various immune-based therapies, including vaccines and checkpoint inhibitors, have shown promising results in the treatment of pancreatic cancer [11]. Numerous studies have focused on these vaccines or checkpoint inhibitors, such as GVAX or PD-1/PD-L1 inhibitors. However, little is known about how tumor-infiltrating immune cells behave in the special microenvironment of PDAC. PDAC has a rich stroma and lacks oxygen, with a blood supply unique from other solid tumors. With these characteristics, immune cell infiltration in this context would be different from that of immune cells isolated from healthy people, as they would be “educated” by the surrounding tumor cells. Consequently, these “educated” immune cells may display impaired functionality.
Typically, a high density of NK cells is correlated with a strong ability to kill “non-self” cells, thus promising robust immunity and improved cancer prognosis. However, our study of a large cohort of 378 advanced PDAC patients revealed a very different result. We unexpectedly found that a high density of CD3−CD16+CD56+ cells, a commonly used surface signature of NK cells, did not guarantee increased killing function in advanced pancreatic cancer. In contrast, increased NK cell levels predicted poor survival in PDAC patients. NLR was also measured. Our results showed that high NLR indicated poor prognosis, similar to previous studies [9, 12, 13]. Together, these data suggest that the NK cells we measured were different from traditional NK cells due to an active inflammatory context, suggestive of a unique subtype with impaired functions. These cells may have been “educated” by tumor cells, resulting in anergy. In fact, the prognosis of PDAC patients can depend on various NK cell subtypes with different functions and phenotypes [14], rather than simple numbers. The inflammation-associated marker NLR revealed an active inflammatory response in advanced PDAC patients. Thus, we assumed that inflammatory response contributed to NK cell anergy.
Questions have emerged regarding the identity of this NK cell subtype and how these cells lose their killing functions in advanced PDAC. We assumed that the lack of killing function was due to insufficient NK cell activation. Activated NK cells produce chemokines and cytokines such as IFN-γ and TNF-α that play an important role in the immune response. Our study found that in PDAC patients, an increased quantity of NK cells was associated with a poor outcome, with the quality of these NK cells reduced. In the poor outcome group, IFN-γ levels were lower than the other group even though the absolute number NK cells was higher (p = 0.0425). Similar results were also found for TNF-α, although the differences were not statistically significant (p = 0.0971). Reductions in IFN-γ and TNF-α reflect impaired NK cell functions, demonstrating that these NK cells are a unique subtype in an anergic state.
Normally, activation of NK cells requires multiple factors, including IL-2, IL-12, IL-15 and IL-18 [15]. IL-2, which is predominately produced by active T cells, plays an important role in regulating NK cell activation [5]. IL-2-deficient mice have impaired NK cell functions [16]. Anergic NK cells were found to regain their functionality after stimulation with IL-2 in vitro [17, 18]. Of note, IL-2 is able to stimulate both cytotoxic effector T cells and Treg cells [18]. Somewhat paradoxically, different doses of IL-2 have different functions. While low doses of IL-2 induce the differentiation of Treg cells [19], high-doses IL-2 have been shown to activate cytotoxic T cells [20]. As IL-2 is one of the cytokines capable of activating NK cells, we hypothesized that NK cell anergy is influenced by reduced levels of IL-2. We analyzed levels of IL-2 in a small sample population. The results showed that in the high NK group, levels of IL-2 were lower. We assumed that a unique T cell distribution influenced by an active inflammatory microenvironment resulted in altered IL-2 secretion, resulting in impaired NK cell activation and disabled functions in this unique subtype. However, the sample size for the IL-2 detection was small, and, therefore, more samples should be enrolled to corroborate these findings.
Several pro-tumorigenic factors secreted by tumor cells can “educate” NK cells as a way to escape attack from the immune system. In melanoma, matrix metalloproteinase-2 (MMP-2) secreted by cancer cells causes NK cells to become exhausted and dysfunctional. Feng et al. showed that MMP-9 and Indoleamine 2,3-dioxygenase (IDO) produced by pancreatic cancer cells result in NK cell anergy, and blockade of MMP-9 and/or IDO restored NK cell function. Other pro-tumorigenic factors that influence cell-extrinsic pathways are other potential factors that can “educate” NK cells, such as eomesodermin and Telomeric repeat binding factor 2 (TRF2). These factors influence NK cell functionality via various mechanisms; for example, MMP-2 degrades the type I IFN receptor 1 and mitigates the phosphorylation of STAT1 [21].
In contrast to NK cells from healthy people, NK cells “educated” by cancer cells bear different phenotypes and show different levels of NK cell receptors [4]. Previous studies have indicated that a subset of NK cells with high levels of PD-1 displayed impaired functions and are defined as CD56dimNKG2A−KIR+CD57+ in ovarian carcinoma [22]. This unique subset lacks the ability to produce cytokines and exert cytotoxic function. We found that when NK cells (from healthy donors) were co-cultured with pancreatic cancer cell lines (SW1990), levels of PD1 were up-regulated, and NK cell functions were impaired (up-regulation of KIR; Supplement Figure 2). These NK cells bear the exhausted phenotype (PD1+). Intratumoral NK cells show an exhausted phenotype. Ndhlovu et al. found that T cell immunoglobulin- and mucin domain-containing (Tim)-3, a marker typically used to identify exhausted T cells, could also indicate NK cell exhaustion [23], and TIM-3 blockade partially reversed NK cell exhaustion [24].
Results from other tumor studies support our hypothesis. Carrega et al. demonstrated that CD56bright NK cells are highly enriched within NSCLC tissues compared to NK cells isolated from autologous peripheral blood, while showing reduced functionality in antitumor responses [25]. A study conducted by Chansac et al. indicated that lung adenocarcinoma cells escape attack from NK cells by downregulating the expression of major histocompatibility complex-1 (MHC-I) [26]. In addition, the authors found that NK cells isolated from healthy donors were able to kill tumor cells, while the tumor-infiltrating NK cells lacked cytotoxic capabilities. During NK cell development, the absence of MHC-I negatively affects the ability of NK cells to kill MHC-I deficient tumor cells [27].
The quantity or quality of NK cells could also be affected by therapeutic interventions. In mice, the numbers of NK cells, but not T cells, are increased upon treatment with gemcitabine following resection of pancreatic tumors [28]. This study also shows that chemotherapy prevents local recurrence by activating the innate immune response. In addition, Plate et al. found that gemcitabine is not immunosuppressive [29]. These findings show that administration of chemotherapy is not harmful to the immune system; rather, it contributes to the activation of the immune response.
The main limitation of our work is the absence of a potential underlying mechanism. In addition, a larger cohort is needed for the detection of cytokines and chemokines to confirm our results. We observed a high NLR value and a high density of NK cells in a cohort of patients with poor prognosis. In a sub-analysis, we reported that in the high-density NK group, the level of IL-2, TNF-α and IFN-γ was lower than the other groups. Related studies revealed a possible relationship between these factors. However, correlation does not equal causation. No molecular mechanism was found to explain the correlation between IL-2 and NK. There has not been a robust study on the potential underlying molecular mechanism to support our hypothesis. Additionally, when and where NK cells become anergic should be a primary focus of future research.
In summary, we found that high numbers of NK cells did not promise better outcomes in a derivation cohort. In contrast, NK cell number was correlated with poor prognosis in advanced pancreatic cancer. These results were confirmed in a validation cohort. High NLR also predicted poor prognosis and demonstrated the reliability of the cohort. We hypothesized that the reason for this phenomenon was that the NK cells we measured were “educated” by tumor cells and had become anergic. This may be due to the influence of tumor cells on NK cell activation through inhibition of IL-2, TNF-α and IFN-γ secretion. Finally, we present the hypothesis that NK cells become anergic and show impaired functionality in the microenvironment of advanced pancreatic cancer.
Abbreviations
- CBC:
-
Complete blood counts
- CI:
-
Confidence interval
- FDUSCC:
-
Fudan University Shanghai Cancer Center
- HIS:
-
Hospital Information System
- HR:
-
Hazard ratio
- MMP:
-
Matrix metalloproteinase
- NCAM:
-
Neural cell adhesion molecule
- NLR:
-
Neutrophil-to-lymphocyte ratio
- PDAC:
-
Pancreatic ductal adenocarcinoma
- TRF2:
-
Telomeric repeat binding factor 2
References
Yang C, Liu C, Yu X (2018) Anergic natural killer cells educated by tumor cells are associated with poor prognosis in patients with advanced pancreatic ductal adenocarcinoma. Pancreatology 18(4 Supplement):S134 (poster number: P3–104)
Siegel RL, Miller KD, Jemal A (2017) Cancer statistics, 2017. CA Cancer J Clin 67:7–30
Xu YF, Lu Y, Cheng H, Shi S, Xu J, Long J, Liu L, Liu C, Yu X (2014) Abnormal distribution of peripheral lymphocyte subsets induced by PDAC modulates overall survival. Pancreatology 14:295–301
Platonova S, Cherfils-Vicini J, Damotte D, Crozet L, Vieillard V, Validire P, Andre P, Dieu-Nosjean MC, Alifano M, Regnard JF, Fridman WH, Sautes-Fridman C, Cremer I (2011) Profound coordinated alterations of intratumoral NK cell phenotype and function in lung carcinoma. Cancer Res 71:5412–5422
Fehniger TA, Cooper MA, Nuovo GJ, Cella M, Facchetti F, Colonna M, Caligiuri MA (2003) CD56bright natural killer cells are present in human lymph nodes and are activated by T cell-derived IL-2: a potential new link between adaptive and innate immunity. Blood 101:3052–3057
Costanzo MC, Creegan M, Lal KG, Eller MA (2015) OMIP-027: functional analysis of human natural killer cells. Cytometry A 87:803–805
Bhatti I, Peacock O, Lloyd G, Larvin M, Hall RI (2010) Preoperative hematologic markers as independent predictors of prognosis in resected pancreatic ductal adenocarcinoma: neutrophil–lymphocyte versus platelet–lymphocyte ratio. Am J Surg 200:197–203
Garcea G, Ladwa N, Neal CP, Metcalfe MS, Dennison AR, Berry DP (2011) Preoperative neutrophil-to-lymphocyte ratio (NLR) is associated with reduced disease-free survival following curative resection of pancreatic adenocarcinoma. World J Surg 35:868–872
Luo G, Guo M, Liu Z, Xiao Z, Jin K, Long J, Liu L, Liu C, Xu J, Ni Q, Yu X (2015) Blood neutrophil–lymphocyte ratio predicts survival in patients with advanced pancreatic cancer treated with chemotherapy. Ann Surg Oncol 22:670–676
Kuzman JA, Stenehjem DD, Merriman J, Agarwal AM, Patel SB, Hahn AW, Alex A, Albertson D, Gill DM, Agarwal N (2017) Neutrophil–lymphocyte ratio as a predictive biomarker for response to high dose interleukin-2 in patients with renal cell carcinoma. BMC Urol 17:1
Foley K, Kim V, Jaffee E, Zheng L (2016) Current progress in immunotherapy for pancreatic cancer. Cancer Lett 381:244–251
Stotz M, Gerger A, Eisner F, Szkandera J, Loibner H, Ress AL, Kornprat P, AlZoughbi W, Seggewies FS, Lackner C, Stojakovic T, Samonigg H, Hoefler G, Pichler M (2013) Increased neutrophil–lymphocyte ratio is a poor prognostic factor in patients with primary operable and inoperable pancreatic cancer. Br J Cancer 109:416–421
Ben Q, An W, Wang L, Wang W, Yu L, Yuan Y (2015) Validation of the pretreatment neutrophil–lymphocyte ratio as a predictor of overall survival in a cohort of patients with pancreatic ductal adenocarcinoma. Pancreas 44:471–477
Balsamo M, Scordamaglia F, Pietra G, Manzini C, Cantoni C, Boitano M, Queirolo P, Vermi W, Facchetti F, Moretta A, Moretta L, Mingari MC, Vitale M (2009) Melanoma-associated fibroblasts modulate NK cell phenotype and antitumor cytotoxicity. Proc Natl Acad Sci USA 106:20847–20852
Kerdiles Y, Ugolini S, Vivier E (2013) T cell regulation of natural killer cells. J Exp Med 210:1065–1068
Kundig TM, Schorle H, Bachmann MF, Hengartner H, Zinkernagel RM, Horak I (1993) Immune responses in interleukin-2-deficient mice. Science 262:1059–1061
Horowitz A, Hafalla JC, King E, Lusingu J, Dekker D, Leach A, Moris P, Cohen J, Vekemans J, Villafana T, Corran PH, Bejon P, Drakeley CJ, von Seidlein L, Riley EM (2012) Antigen-specific IL-2 secretion correlates with NK cell responses after immunization of Tanzanian children with the RTS,S/AS01 malaria vaccine. J Immunol 188:5054–5062
Boyman O, Sprent J (2012) The role of interleukin-2 during homeostasis and activation of the immune system. Nat Rev Immunol 12:180–190
Saadoun D, Rosenzwajg M, Joly F, Six A, Carrat F, Thibault V, Sene D, Cacoub P, Klatzmann D (2011) Regulatory T-cell responses to low-dose interleukin-2 in HCV-induced vasculitis. N Engl J Med 365:2067–2077
Boyman O, Kovar M, Rubinstein MP, Surh CD, Sprent J (2006) Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science 311:1924–1927
Lee BK, Kim MJ, Jang HS, Lee HR, Ahn KM, Lee JH, Choung PH, Kim MJ (2008) A high concentration of MMP-2/gelatinase A and MMP-9/gelatinase B reduce NK cell-mediated cytotoxicity against an oral squamous cell carcinoma cell line. In Vivo 22:593–597
Pesce S, Greppi M, Tabellini G, Rampinelli F, Parolini S, Olive D, Moretta L, Moretta A, Marcenaro E (2017) Identification of a subset of human natural killer cells expressing high levels of programmed death 1: a phenotypic and functional characterization. J Allergy Clin Immunol 139:335–346
Ndhlovu LC, Lopez-Verges S, Barbour JD, Jones RB, Jha AR, Long BR, Schoeffler EC, Fujita T, Nixon DF, Lanier LL (2012) Tim-3 marks human natural killer cell maturation and suppresses cell-mediated cytotoxicity. Blood 119:3734–3743
Gonzalez-Gugel E, Saxena M, Bhardwaj N (2016) Modulation of innate immunity in the tumor microenvironment. Cancer Immunol Immunother 65:1261–1268
Carrega P, Morandi B, Costa R, Frumento G, Forte G, Altavilla G, Ratto GB, Mingari MC, Moretta L, Ferlazzo G (2008) Natural killer cells infiltrating human nonsmall-cell lung cancer are enriched in CD56 bright CD16(−) cells and display an impaired capability to kill tumor cells. Cancer 112:863–875
Le Maux CB, Moretta A, Vergnon I, Opolon P, Lecluse Y, Grunenwald D, Kubin M, Soria JC, Chouaib S, Mami-Chouaib F (2005) NK cells infiltrating a MHC class I-deficient lung adenocarcinoma display impaired cytotoxic activity toward autologous tumor cells associated with altered NK cell-triggering receptors. J Immunol 175:5790–5798
Bix M, Liao NS, Zijlstra M, Loring J, Jaenisch R, Raulet D (1991) Rejection of class I MHC-deficient haemopoietic cells by irradiated MHC-matched mice. Nature 349:329–331
Gurlevik E, Fleischmann-Mundt B, Brooks J, Demir IE, Steiger K, Ribback S, Yevsa T, Woller N, Kloos A, Ostroumov D, Armbrecht N, Manns MP, Dombrowski F, Saborowski M, Kleine M, Wirth TC, Oettle H, Ceyhan GO, Esposito I, Calvisi DF, Kubicka S, Kuhnel F (2016) Administration of gemcitabine after pancreatic tumor resection in mice induces an antitumor immune response mediated by natural killer cells. Gastroenterology 151:338–350
Plate JM, Plate AE, Shott S, Bograd S, Harris JE (2005) Effect of gemcitabine on immune cells in subjects with adenocarcinoma of the pancreas. Cancer Immunol Immunother 54:915–925
Funding
This work was supported by National Natural Science Foundation of China Grants (81370065, 81372653), a basic research project of the Science and Technology Commission of Shanghai Municipality (15JC1401200), and the National Science Fund for Distinguished Young Scholars (81625016).
Author information
Authors and Affiliations
Contributions
CY, HC, CL and XY were involved in the study conception and design. CY and HC were involved in the acquisition, analysis, and interpretation of the data. CY drafted the manuscript, and all authors were involved in critical revision of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest to disclose.
Ethical approval and ethical standards
The study was approved by the Ethics Committee of Fudan University Shanghai Cancer Center (FDUSCC) and met ethical guidelines of the World Medical Association Declaration of Helsinki. Written informed consent was acquired at FDUSCC.
Additional information
The work was published as a poster at the 50th Jubilee Meeting of the European Pancreatic Club (EPC2018), 13–16 June 2018, in Berlin, Germany [1].
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yang, C., Cheng, H., Zhang, Y. et al. Anergic natural killer cells educated by tumor cells are associated with a poor prognosis in patients with advanced pancreatic ductal adenocarcinoma. Cancer Immunol Immunother 67, 1815–1823 (2018). https://doi.org/10.1007/s00262-018-2235-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-018-2235-8