Abstract
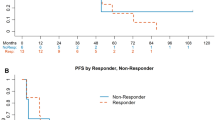
MUC1 over-expression in renal clear-cell carcinoma (RCC) is associated with poor prognosis. This phase II study determined the efficacy and tolerability of TG4010, a cancer vaccine based on a modified vaccinia virus expressing MUC1 and interleukin-2, in combination with cytokines, as first-line therapy in metastatic RCC. Thirty-seven patients with progressive, MUC1-positive RCC received TG4010 108 pfu/inj weekly for 6 weeks, then every 3 weeks until progression, when TG4010 was continued in combination with interferon-α2a and interleukin-2. Assessments included clinical response (primary endpoint), safety, time to treatment failure (TTF), overall survival (OS), and immune response. No objective clinical responses occurred. Five of the 27 evaluable patients (18%) had stable disease for >6 months with TG4010 alone and six of 20 patients (30%) had stable disease for >6 months with TG4010 plus cytokines. Median TTF was 4.1, 3.6, and 9.3 months for monotherapy, combination therapy, and overall, respectively. Median OS was 19.3 months for all patients and 22.4 months combination therapy recipients. The most frequent TG4010-related adverse events were minor-to-moderate injection-site reactions, fatigue, and flu-like symptoms. Six of 28 patients showed a MUC1 CD4+ T cell proliferative response during therapy. Anti-MUC1 CD8+ T cells were detected before and after therapy in 3 and 4 patients, respectively. MUC1-specific CD8+ T cell responses were associated with longer survival. Therapy with TG4010 plus cytokines appears to be feasible and well tolerated in patients with metastatic RCC. However, these data should be interpreted with caution, as additional prospective studies are necessary to clarify the clinical efficacy of this therapy.



Similar content being viewed by others
References
Wesseling J, van der Valk SW, Vos HL et al (1995) Episialin (MUC1) overexpression inhibits integrin-mediated cell adhesion to extracellular matrix components. J Cell Biol 129:255–265
Brayman M, Thathiah A, Carson DD (2004) MUC1: a multifunctional cell surface component of reproductive tissue epithelia. Reprod Biol Endocrinol 2:4
Hilkens J, Buijs F, Hilgers J et al (1984) Monoclonal antibodies against human milk-fat globule membranes detecting differentiation antigens of the mammary gland and its tumors. Int J Cancer 34:197–206
Leroy X, Zerimech F, Zini L et al (2002) MUC1 expression is correlated with nuclear grade and tumor progression in pT1 renal clear cell carcinoma. Am J Clin Pathol 118:47–51
Kraus S, Abel PD, Nachtmann C et al (2002) MUC1 mucin and trefoil factor 1 protein expression in renal cell carcinoma: correlation with prognosis. Hum Pathol 33:60–67
Brossart P, Heinrich KS, Stuhler G et al (1999) Identification of HLA-A2-restricted T-cell epitopes derived from the MUC1 tumor antigen for broadly applicable vaccine therapies. Blood 93:4309–4317
Goydos JS, Elder E, Whiteside TL et al (1996) A phase I trial of a synthetic mucin peptide vaccine. Induction of specific immune reactivity in patients with adenocarcinoma. J Surg Res 63:298–304
Apostolopoulos V, Sandrin MS, McKenzie IF (1999) Carbohydrate/peptide mimics: effect on MUC1 cancer immunotherapy. J Mol Med 77:427–436
Miles D, Papazisis K (2003) Rationale for the clinical development of STn-KLH (Theratope) and anti-MUC-1 vaccines in breast cancer. Clin Breast Cancer 3(Suppl 4):S134–S138
Pantuck AJ, van Ophoven A, Gitlitz BJ et al (2004) Phase I trial of antigen-specific gene therapy using a recombinant vaccinia virus encoding MUC-1 and IL-2 in MUC-1-positive patients with advanced prostate cancer. J Immunother 27:240–253
Rochlitz C, Figlin R, Squiban P et al (2003) Phase I immunotherapy with a modified vaccinia virus (MVA) expressing human MUC1 as antigen-specific immunotherapy in patients with MUC1-positive advanced cancer. J Gene Med 5:690–699
Karanikas V, Hwang LA, Pearson J et al (1997) Antibody and T cell responses of patients with adenocarcinoma immunized with mannan-MUC1 fusion protein. J Clin Invest 100:2783–2792
Ramlau R, Quoix E, Rolski J et al (2008) A phase II study of Tg4010 (MVA–MUC1–IL2) in association with chemotherapy in patients with stage III/IV non-small cell lung cancer. J Thorac Oncol 3:735–744
Keydar I, Chou CS, Hareuveni M et al (1989) Production and characterization of monoclonal antibodies identifying breast tumor-associated antigens. Proc Natl Acad Sci USA 86:1362–1366
Mayr A, Hochstein-Mintzel V, Stickl H (1975) Abstammung, Eigenschaften und Verwendung des attenuirten Vaccinia-Stammes MVA. Infection 3:6–14
Stickl H, Hochstein-Mintzel V, Mayr A et al (1974) MVA vaccination against smallpox: clinical tests with an attenuated live vaccinia virus strain (MVA). Dtsch Med Wochenschr 99:2386–2392 (author’s translation)
Mayr A, Stickl H, Muller HK et al (1978) The smallpox vaccination strain MVA: marker, genetic structure, experience gained with the parenteral vaccination and behavior in organisms with a debilitated defence mechanism. Zentralbl Bakteriol B 167:375–390 (author’s translation)
Meyer H, Sutter G, Mayr A (1991) Mapping of deletions in the genome of the highly attenuated vaccinia virus MVA and their influence on virulence. J Gen Virol 72(Pt 5):1031–1038
Sutter G, Moss B (1992) Nonreplicating vaccinia vector efficiently expresses recombinant genes. Proc Natl Acad Sci USA 89:10847–10851
Tourani JM, Pfister C, Tubiana N et al (2003) Subcutaneous interleukin-2 and interferon alfa administration in patients with metastatic renal cell carcinoma: final results of SCAPP III, a large, multicenter, phase II, nonrandomized study with sequential analysis design—the Subcutaneous Administration Propeukin Program Cooperative Group. J Clin Oncol 21:3987–3994
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
National Cancer Institute (1999) Common toxicity criteria (CTC), version 2.0. Available at http://ctep.cancer.gov/protocol/development/electronic_applications/docs/ctcv20_4-30-992.pdf
Franzke A, Peest D, Probst-Kepper M et al (1999) Autoimmunity resulting from cytokine treatment predicts long-term survival in patients with metastatic renal cell cancer. J Clin Oncol 17:529–533
Bercovici N, Haicheur N, Massicard S et al (2008) Analysis and characterization of antitumor T-cell response after administration of dendritic cells loaded with allogeneic tumor lysate to metastatic melanoma patients. J Immunother 31:101–112
Godard B, Gazagne A, Gey A et al (2004) Optimization of an elispot assay to detect cytomegalovirus-specific CD8 + T lymphocytes. Hum Immunol 65:1307–1318
Wang XF, Kerzerho J, Adotevi O et al (2008) Comprehensive analysis of HLA-DR- and HLA-DP4-restricted CD4+ T cell response specific for the tumor-shared antigen survivin in healthy donors and cancer patients. J Immunol 181:431–439
Heukamp LC, van der Burg SH, Drijfhout JW et al (2001) Identification of three non-VNTR MUC1-derived HLA-A*0201-restricted T-cell epitopes that induce protective anti-tumor immunity in HLA-A2/K(b)-transgenic mice. Int J Cancer 91:385–392
van der Burg SH, Ras E, Drijfhout JW et al (1995) An HLA class I peptide-binding assay based on competition for binding to class I molecules on intact human B cells. Identification of conserved HIV-1 polymerase peptides binding to HLA-A*0301. Hum Immunol 44:189–198
Van Poppel H, Joniau S, Van Gool SW (2009) Vaccine therapy in patients with renal cell carcinoma. Eur Urol 55:1333–1342
Negrier S, Escudier B, Lasset C et al (1998) Recombinant human interleukin-2, recombinant human interferon alfa-2a, or both in metastatic renal-cell carcinoma. Groupe Francais d’Immunotherapie. N Engl J Med 338:1272–1278
Fujita K, Denda K, Yamamoto M et al (1999) Expression of MUC1 mucins inversely correlated with post-surgical survival of renal cell carcinoma patients. Br J Cancer 80:301–308
Benchetrit F, Gazagne A, Adotevi O et al (2003) Cytotoxic T lymphocytes: role in immunosurveillance and in immunotherapy. Bull Cancer 90:1–9
Lonchay C, van der Bruggen P, Connerotte T et al (2004) Correlation between tumor regression and T cell responses in melanoma patients vaccinated with a MAGE antigen. Proc Natl Acad Sci USA 101(Suppl 2):14631–14638
Pages F, Berger A, Camus M et al (2005) Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med 353:2654–2666
Reichardt VL, Okada CY, Liso A et al (1999) Idiotype vaccination using dendritic cells after autologous peripheral blood stem cell transplantation for multiple myeloma—a feasibility study. Blood 93:2411–2419
Scholfield DP, Simms MS, Bishop MC (2003) MUC1 mucin in urological malignancy. BJU Int 91:560–566
Wierecky J, Muller MR, Wirths S et al (2006) Immunologic and clinical responses after vaccinations with peptide-pulsed dendritic cells in metastatic renal cancer patients. Cancer Res 66:5910–5918
Amato RJ, Shingler W, Naylor S et al (2008) Vaccination of renal cell cancer patients with modified vaccinia ankara delivering tumor antigen 5T4 (TroVax) administered with interleukin 2: a phase II trial. Clin Cancer Res 14:7504–7510
Rosenberg SA, Yang JC, Schwartzentruber DJ et al (1998) Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat Med 4:321–327
Sosman JA, Carrillo C, Urba WJ et al (2008) Three phase II cytokine working group trials of gp100 (210M) peptide plus high-dose interleukin-2 in patients with HLA-A2-positive advanced melanoma. J Clin Oncol 26:2292–2298
Josefowicz SZ, Rudensky A (2009) Control of regulatory T cell lineage commitment and maintenance. Immunity 30:616–625
Setoguchi R, Hori S, Takahashi T et al (2005) Homeostatic maintenance of natural Foxp3(+) CD25(+) CD4(+) regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. J Exp Med 201:723–735
Ahmadzadeh M, Rosenberg SA (2006) IL-2 administration increases CD4+ CD25(hi) Foxp3+ regulatory T cells in cancer patients. Blood 107:2409–2414
van der Vliet HJ, Koon HB, Yue SC et al (2007) Effects of the administration of high-dose interleukin-2 on immunoregulatory cell subsets in patients with advanced melanoma and renal cell cancer. Clin Cancer Res 13:2100–2108
Lechleider RJ, Arlen PM, Tsang KY et al (2008) Safety and immunologic response of a viral vaccine to prostate-specific antigen in combination with radiation therapy when metronomic-dose interleukin 2 is used as an adjuvant. Clin Cancer Res 14:5284–5291
Kaufman HL, Taback B, Sherman W et al (2009) Phase II trial of Modified Vaccinia Ankara (MVA) virus expressing 5T4 and high dose Interleukin-2 (IL-2) in patients with metastatic renal cell carcinoma. J Transl Med 7:2
Cesana GC, DeRaffele G, Cohen S et al (2006) Characterization of CD4+ CD25+ regulatory T cells in patients treated with high-dose interleukin-2 for metastatic melanoma or renal cell carcinoma. J Clin Oncol 24:1169–1177
Rosenberg SA, Yang JC, Restifo NP (2004) Cancer immunotherapy: moving beyond current vaccines. Nat Med 10:909–915
Higano CS, Schellhammer PF, Small EJ et al (2009) Integrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer 115:3670–3679
Kantoff PW, Schuetz TJ, Blumenstein BA et al (2010) Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol 28:1099–1105
Mittendorf EA, Holmes JP, Ponniah S et al (2008) The E75 HER2/neu peptide vaccine. Cancer Immunol Immunother 57:1511–1521
Griffiths RW, Elkord E, Gilham DE et al (2007) Frequency of regulatory T cells in renal cell carcinoma patients and investigation of correlation with survival. Cancer Immunol Immunother 56:1743–1753
Ko JS, Zea AH, Rini BI et al (2009) Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res 15:2148–2157
Badoual C, Sandoval F, Pere H et al (2010) Better understanding tumor-host interaction in head and neck cancer to improve the design and development of immunotherapeutic strategies. Head Neck 32:946–958
Finke JH, Rini B, Ireland J et al (2008) Sunitinib reverses type-1 immune suppression and decreases T-regulatory cells in renal cell carcinoma patients. Clin Cancer Res 14:6674–6682
Ozao-Choy J, Ma G, Kao J et al (2009) The novel role of tyrosine kinase inhibitor in the reversal of immune suppression and modulation of tumor microenvironment for immune-based cancer therapies. Cancer Res 69:2514–2522
Adotevi O, Pere H, Ravel P et al (2010) A decrease of regulatory CD4+CD25hiFoxp3+ T cells correlate with clinical response after sunitinib based anti-angiogenic therapy regimen in metastatic renal cancer patients J Immunother (in press)
Xin H, Zhang C, Herrmann A et al (2009) Sunitinib inhibition of Stat3 induces renal cell carcinoma tumor cell apoptosis and reduces immunosuppressive cells. Cancer Res 69:2506–2513
Vingert B, Adotevi O, Patin D et al (2006) The Shiga toxin B-subunit targets antigen in vivo to dendritic cells and elicits anti-tumor immunity. Eur J Immunol 36:1124–1135
Adotevi O, Vingert B, Freyburger L et al (2007) B Subunit of shiga toxin-based vaccines synergize with {alpha}-galactosylceramide to break tolerance against self antigen and elicit antiviral immunity. J Immunol 179:3371–3379
Geiger C, Nossner E, Frankenberger B et al (2009) Harnessing innate and adaptive immunity for adoptive cell therapy of renal cell carcinoma. J Mol Med 87:595–612
Acknowledgments
We thank Andrea Bothwell and Natalie Carter of inScience Communications, a Wolters Kluwer business, who provided copy editing and journal styling prior to and post submission. This work was supported by Ligue contre le Cancer; Pôle de compétitivité Medicen (Immucan); Canceropole d’Ile de France; Centre d’Investigation Clinique en Biotherapie. Assistance Publique des Hôpitaux de Paris (CIC-BT AP-HP).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Oudard, S., Rixe, O., Beuselinck, B. et al. A phase II study of the cancer vaccine TG4010 alone and in combination with cytokines in patients with metastatic renal clear-cell carcinoma: clinical and immunological findings. Cancer Immunol Immunother 60, 261–271 (2011). https://doi.org/10.1007/s00262-010-0935-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-010-0935-9