Abstract
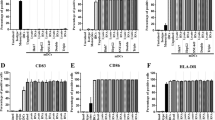
Dendritic cells (DCs) are one of the most potent antigen-presenting cells (APCs) capable of activating immune responses. Different forms of tumor antigens have been used to load DCs to initiate tumor-specific immune responses. Heat shock proteins (HSPs) are considered natural adjuvants which have the ability to chaperone peptides associated with them presented efficiently by interaction with professional APCs through specific receptors. In the present study, we used HSP, gp96-peptide complexes, derived from human hepatocellular carcinoma (HCC) cells as antigens for pulsing DCs. We found that gp96-peptide complexes derived from HCC cells induced the maturation of DCs by enhancing expression of human leukocyte antigen class II, CD80, CD86, CD40, and CD83. The matured DCs stimulated a high level of autologous T cell proliferation and induced HCC specific cytotoxic T lymphocytes, which specifically killed HCC cells by a major histocompatability complex (MHC) class I restricted mechanism. These findings demonstrate that DCs pulsed with gp96-peptide complexes derived from HCC cells are effective in activating specific T cell responses against HCC cells.




Similar content being viewed by others
References
Arnold D, Faath S, Rammensee HG, Schild H (1995) Cross-priming of minor-histocompatibility antigen specific cytotoxic T cells upon immunization with the heat-shock protein gp96. J Exp Med 182:885–889
Arnold-Schild D, Hanau D, Spehner D, Schmid D, Rammensee HG, de la Salle H, Schild H (1999) Cutting edge: receptor-mediated endocytosis of heat shock proteins by professional antigen-presenting cells. J Immunol 162:3757–3760
Ashley DM, Faiola B, Nair S, Hale LP, Bigner DD, Gilboa E (1997) Bone marrow-generated dendritic cells pulsed with tumor extracts or tumor RNA induce antitumor immunity against central nervous system tumors. J Exp Med 186:1177–1182
Baar J (1999) Clinical applications of dendritic cell cancer vaccines. Oncologist 4:140–144
Baker-LePain JC, Sarzotti M, Fields TA, Li CY, Nicchitta CV (2002) GRP94(gp96) and GRP94 N-terminal geldanamycin binding domain elicit tissue nonrestricted tumor suppression. J Exp Med 196:1447–1459
Banchereau J, Steinman RM (1998) Dendritic cells and the control of immunity. Nature 392:245–252
Basu S, Binder RJ, Ramalingam T, Srivastava PK (2001) CD91 is a common receptor for heat shock proteins gp96, hsp90, hsp70, and calreticulin. Immunity 14:303–313
Basu S, Binder RJ, Suto R, Anderson KM, Srivastava PK (2000) Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kB pathway. Int Immunol 12:1539–1546
Basu S, Srivastava PK (2000) Heat shock proteins: the fountainhead of innate and adaptive immune responses. Cell Stress Chaperones 5: 443–451
Berwin B, Hart JP, Pizze SV, Nicchitta CV (2002) Cutting edge: CD91-independent cross-presentation of GRP94 (gp96)-associated peptides. J Immunol 168: 4282–4286
Bodey B, Bodey B Jr, Siegel SE, Kaiser HE (2000) Failure of cancer vaccines: the significant limitations of this approach to immunotherapy. Anticancer Res 20: 2665–2676
Castelli C, Ciupitu AM, Rini F, Rivoltini L, Mazzocchi A, Kiessling R, Parmiani G (2001) Human heat shock protein 70 peptide complexes specially activate antimelanoma T cells. Cancer Res 61:222–227
Cavallo F, Martin-Fontecha A, Bellone M, Heltai S, Gatti E, Tornaghi P, Freschi M, Forni G, Dellabona P, Casorati G (1995) Co-expression of B7–1 and ICAM-1 on tumors is required for rejection and the establishment of a memory response. Eur J Immunol 25:1154–1162
Cho KB, Palliser D, Guillen E, Wisniewski J, Young RA, Chen JZ, Eisen HN (2000) A proposed mechanism for the induction of cytotoxic T lymphocyte production by heat shock fusion proteins. Immunity 12:263–272
Dhodapkar MV, Bhardwaj N (2000) Active immunization of humans with dendritic cells. J Clin Immunol 20:167–174
Dong R (1989) Establishment of a human hepatocarcinoma cell line SMMC-7721 and initial observations on its biologic characteristics. In: Primary liver cancer. China Academic Publishers, Beijing, Springer, Berlin Heidelburg New York, pp145–153
Ferrone S, Finerty JF, Jaffee EM, Nabel GJ (2000) How much longer will tumor cells fool the immune system? Immunol Today 21:70–72
Gong JL, Nikrui N, Chen DS, Koido S, Wu ZK, Tanaka Y, Cannistra S, Avigan D, Kufe D (2000) Fusions of human ovarian carcinoma cells with autologous or allogeneic dendritic cells induce antitumor immunity. J Immunol 165:1705–1711
Hajek R, Butch AW (2000) Dendritic cell biology and the application of dendritic cells to immunotherapy of multiple myeloma. Med Oncol 17:2–15
Janetzki S, Blachere NE, Srivastava PK (1998) Generation of tumor-specific cytotoxic T lymphocytes and memory T cells by immunization with tumor-derived heat shock protein gp96. J Immunother 21:269–276
Janetzki S, Palla D, Rosenhauer V, Lochs H, Lewis JJ, Srivastava PK (2000) Immunization of cancer patients with autologous cancer-derived heat shock protein gp96 preparations: a pilot study. Int J Cancer 88:232–238
Lindquist S, Craig E (1988) The heat-shock proteins. Annu Rev Genet 22:631–677
Liu BB, Ye SL, He P, Liu YK, Tang ZY (1999) MAGE-1 and related MAGE gene expression may be associated with hepatocellular carcinoma. J Cancer Res Clin Oncol 125:685–689
Mazzaferro V, Coppa J, Carrabba MG, Rivoltini L, Schiavo M, Regalia E, Mariani L, Camerini T, Marchiano A, Andreola S, Camerini R, Corsi M, Lewis JJ, Srivastava PK, Parmiani G (2003) Vaccination with autologous tumor-derived heat-shock protein gp96 after liver resection for metastatic colorectal cancer. Clin Cancer Res 9:3235–3241
Murakami M, Gurski KJ, Marincola FM, Ackland J, Steller MA (1999) Induction of specific CD8+ T-lymphocyte responses using a human papillomavirus-16 E6/E7 fusion protein and autologous dendritic cells. Cancer Res 59(6):1184–1187
Ota S, Ono T, Morita A, Uenaka A, Harada M, Nakayama E (2002) Cellular processing of a multibranched lysine core with tumor antigen peptides and presentation of peptide epitopes recognized by cytotoxic T lymphocytes on antigen-presenting cells. Cancer Res 62(5):1471–1476
Pandey M, Mathew A, Nair MK (1999) Cancer vaccines: a step towards prevention and treatment of cancer. Eur J Surg Oncol 25:209–214
Pockley AG (2003) Heat shock proteins as regulators of the immune response. Lancet 362:469–476
Rivoltini L, Castelli C, Carrabba M, Mazzaferro V, Pilla L, Huber V, Coppa J, Gallino G, Scheibenbogen C, Squarcina P, Cova A, Camerini R, Lewis JJ, Srivastava PK, Parmiani G (2003) Human tumor-derived heat shock protein 96 mediates in vitro activation and in vivo expansion of melanoma- and colon carcinoma specific T cells. J Immunol 171:3467–3474
Singh-Jasuja H, Scherer HU, Hilf N, Arnold-Schild D, Rammensee HG, Toes RE, Schild H (2000) The heat shock protein gp96 induces maturation of dendritic cells and down-regulation of its receptor. Eur J Immunol 30:2211–2215
Singh-Jasuja H, Toes RE, Spee P, Munz C, Hilf N, Schoenberger SP, Ricciardi-Castagnoli P, Neefjes J, Rammensee HG, Arnold-Schild D, Schild H (2000) Cross-presentation of glycoprotein 96-associated antigens on major histocompatibility complex class I molecules requires receptor-mediated endocytosis. J Exp Med 191:1965–1974
Srivastava PK (1997) Purification of heat shock protein-peptide complexes for use in vaccination against cancers and intracellular pathogens. Methods 12:165–171
Srivastava PK, Amato RJ (2001) Heat shock proteins: the ‘swiss army knife’ vaccines against cancers and infectious agents. Vaccine 19:2590–2597
Srivastava PK, Jaikaria NS (2000) Methods of purification of heat shock protein-peptide complexes for use as vaccines against cancers and infectious diseases. Methods Mol Biol 156:175–186
Suto R, Srivastava PK (1995) A mechanism for the specific immunogenicity of heat shock protein-chaperoned peptides. Science 269:1585–1588
Suzue K, Zhou XZ, Eisen HN, Yong RA (1997) Heat shock fusion proteins as vehicles for antigen delivery into the major histocompatibility complex class I presentation pathway. Proc Natl Acad Sci 94:13146–13151
Takayama T, Sekine T, Makuuchi M, Yamasaki S, Kosuge T, Yamamoto J, Shimada K, Sakamoto M, Hirohashi S, Ohashi Y, Kakizoe T (2000) Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: a randomized trial. Lancet 356:802–807
Tamura Y, Peng P, Liu K, Daou M, Srivastava PK (1997) Immunotherapy of tumors with autologous tumor-derived heat shock protein preparations. Science 278:117–120
Thurner B, Roder C, Dieckmann D, Heuer M, Kruse M, Glaser A, Keikavoussi P, Kampgen E, Bender A, Schuler G (1999) Generation of large numbers of fully mature and stable dendritic cells from leukapheresis products for clinical application. J Immunol Methods 223:1–15
Vabulas RM, Braedel S, Hilf N, Singh-Jasuja H, Herter S, Ahmad-Nejad P, Kirschning CJ, Costa C, Rammensee HG, Wagner H, Schild H (2002) The endoplasmic reticulum-resident heat shock protein gp96 activates dendritic cells via the Toll-like receptor 2/4 pathway. J Biol Chem 277:20847–20853
Yang S, Kittlesen D, Slingluff CL Jr, Vervaert CE, Seigler HF, Darrow TL (2000) Dendritic cells infected with a vaccinia vector carrying the human gp100 gene simultaneously present multiple specificities and elicit high-affinity T cells reactive to multiple epitopes and restricted by HLA-A2 and -A3. J Immunol 164(8):4204–4211
Yedavelli SPK, Guo L, Daou ME, Srivastava PK, Mittelman, Tiwari PK (1999) Preventive and therapeutic effect of tumor derived heat shock protein, gp96, in an experimental prostate cancer model. Int J Mol Med 4:243–248
Zhang X, Liu DQ, Liu YZ, Wang P, Chen XY (2000). Preliminary study on apoptosis of BEL-7402 cells induced by Chinese herbs for warming yang and dispersing stasis. Zhongguo Zhong Yao Za Zhi/China J Chin Mater Med 25(7):428–430
Zitvogel L, Mayordomo JI, Tjandrawan T, Deleo AB, Clarke MR, Lotze MT, Storkus WJ (1996) Therapy of murine tumors with tumor peptide-pulsed dendritic cells: dependence on T cells, B7 costimulation, and T helper cell 1-associated cytokines. J Exp Med 183:87–97
Acknowledgments
We thank Beijing Red Cross Blood Center for supplying blood samples. We also thank the FACS laboratory of Peking University Health Science Centre for technical support in performing FACS analysis. We appreciate Professor Tian Bo (Institute of Microbiology, Chinese Academy of Sciences) for giving us the plasmid pET30a-gp96.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, X.H., Qin, Y., Hu, M.H. et al. Dendritic cells pulsed with gp96-peptide complexes derived from human hepatocellular carcinoma (HCC) induce specific cytotoxic T lymphocytes. Cancer Immunol Immunother 54, 971–980 (2005). https://doi.org/10.1007/s00262-005-0662-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-005-0662-9