Abstract
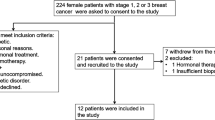
Adjuvant treatment is still only working in a small percentage of breast cancer patients. Therefore, new strategies need to be developed. Immunotherapies are a very promising approach because they could successfully attack tumor cells in the stage of dormancy. To assess the feasibility of using an allogeneic approach for vaccination of breast cancer patients, we selected a CD80-transfected breast cancer cell line based on its immunogenic properties. Using CD80+ KS breast cancer cells and human leukocyte antigen (HLA)-A*02–matched peripheral blood mononuclear cells (PBMCs) of breast cancer patients in allogeneic mixed lymphocyte–tumor cell cultures (MLTCs), it was possible to isolate HLA-A*02–restricted cytotoxic T cells (CTLs). Furthermore, a genetically modified KS variant expressing influenza A matrix protein serving as a surrogate tumor-associated antigen (TAA) was able to stimulate flu peptide-specific T cells alongside the induction of alloresponses in MLTCs. KS breast cancer cells were demonstrated to express already known TAAs such as CEA, MUC-1, MAGE-1, MAGE-2, and MAGE-3. To further improve antigenicity, HER-2/neu was added to this panel as a marker antigen known to elicit HLA-A*02–restricted CTLs in patients with breast cancer. Thus, the antigen-processing and antigen-presentation capacity of KS cells was further demonstrated by the stimulation of HER-2/neu–specific CD8+ T cells in PBMCs of breast cancer patients in vitro. These results gave a good rationale for a phase I/II trial, where the CD80+ HER-2/neu–overexpressing KS variant is actually used as a cellular vaccine in patients with metastatic breast cancer. As a proof of principle, we present data from two patients where a significant increase of interferon-γ (IFN-γ) release was detected when postvaccination PBMCs were stimulated by allogeneic vaccine cells as well as by HLA-A*02–restricted HER-2/neu epitopes. In whole cell vaccine trials, monitoring is particularly challenging because of strong alloresponses and limited knowledge of TAAs. In this study, a panel of HER-2/neu epitopes, together with the quantitative real time (qRT)-PCR method to analyze vaccine-induced cytokines secreted by T cells, proved to be highly sensitive and feasible to perform an “immunological staging” following vaccination.




Similar content being viewed by others
References
Arienti F, Belli F, Napolitano F, Sule-Suso J, Mazzocchi A, Gallino GF, Cattelan A, Santantonio C, Rivoltini L, Melani C, Colombo MP, Cascinelli N, Maio M, Parmiani G, Sanantonio C (2000) Vaccination of melanoma patients with interleukin 4 gene-transduced allogeneic melanoma cells. Hum Gene Ther 10:2907–2916
Baskar S, Nabavi N, Glimcher LH, Ostrand-Rosenberg S (1993) Tumor cells expressing major histocompatibility complex class II and B7 activation molecules stimulate potent tumor-specific immunity. J Immunother 14:209–215
Baxevanis CN, Sotiropoulou PA, Sotiriadou NN, Papamichail M (2004) Immunobiology of Her-2/neu oncoprotein and its potential application in cancer immunotherapy. Cancer Immunol Immunother 53:166–175
Bednarek MA, Engl SA, Gammon MC, Lindquist JA, Porter G, Williamson AR, Zweerink HJ (1991) Soluble HLA-A2.1 restricted peptides that are recognized by influenza virus specific cytotoxic T lymphocytes. J Immunol Methods 139:41–47
Brossart P, Heinrich KS, Stuhler G, Behnke L, Reichardt VL, Stevanovic S, Muhm A, Rammensee HG, Kanz L, Brugger W (1999) Identification of HLA-A2-restricted T-cell epitopes derived from the MUC1 tumor antigen for broadly applicable vaccine therapies. Blood 93:4309–4317
Cayeux S, Beck C, Aicher A, Dorken B, Blankenstein T (1995) Tumor cells cotransfected with interleukin-7 and B7.1 genes induce CD25 and CD28 on tumor-infiltrating T lymphocytes and are strong vaccines. Eur J Immunol 25:2325–2331
Chen L, Ashe S, Brady WA, Hellstrom I, Hellstrom KE, Ledbetter JA, McGowan P, Linsley PS (1992) Costimulation of antitumor immunity by the B7 counterreceptor for the T lymphocyte molecules CD28 and CTLA-4. Cell 71:1093–1102
Courmier JN, Panelli MC, Hackett JA, Bettinotti MP, Mixon A, Wunderlich J, Parker LL, Restifo NP, Ferrone S, Marincola FM (1999) Natural variation of the expression of HLA and endogenous antigen modulates CTL recognition in an in vitro melanoma model. Int J Cancer 80:781–790
Crowley NJ, Slingluff CL Jr, Vervaert CE, Darrow TL, Seigler HF (1990) Inhibition of the growth of human melanoma xenografts in nude mice by human tumor-specific cytotoxic T-cells. J Surg Oncol 43:67–72
Deuschle U, Pepperkok R, Wang FB, Giordano TJ, McAllister WT, Ansorge W, Bujard H (1989) Regulated expression of foreign genes in mammalian cells under the control of coliphage T3 RNA polymerase and lac repressor. Proc Natl Acad Sci U S A 86(14):5400–5404
Dols A, Meijer SL, Smith JW II, Fox BA, Urba WJ (2003) Allogeneic breast cancer cell vaccines. Clin Breast Cancer 3[Suppl 4]:173–180
Dols A, Smith JW II, Meijer SL, Fox BA, Hu HM, Walker E, Rosenheim S, Moudgil T, Doran T, Wood W, Seligman M, Alvord WG, Schoof D, Urba WJ (2003) Vaccination of women with metastatic breast cancer, using a costimulatory gene (CD80)-modified, HLA-A2-matched, allogeneic, breast cancer cell line: clinical and immunological results. Hum Gene Ther 14:1117–1123
Dols A, Meijer SL, Hu HM, Goodell V, Disiss ML, von Mensdorff-Pouilly S, Verheijen R, Alvord WG, Smith JW II, Urba WJ, Fox BA (2003) Identification of tumor-specific antibodies in patients with breast cancer vaccinated with gene-modified allogeneic tumor cells. J Immunother 26:163–170
Fenton RG, Turcovski-Corrales SM, Taub DD (1998) Induction of melanoma antigen-specific cytotoxic T lymphocytes in vitro by stimulation with B7-expressing human melanoma cell lines. J Immunother 21:95–108
Fisk B, Blevins TL, Wharton JT, Ioannides CG (1995) Identification of an immunodominant peptide of Her-2/neu protooncogene recognized by ovarian tumor-specific cytotoxic T lymphocyte lines. J Exp Med 181:2109–2117
Fisk B, Hudson JM, Kavanagh J, Wharton JT, Murray JL, Ioannides CG, Kudelka AP (1997) Existent proliferative response of peripheral blood mononuclear cells from healthy donors and ovarian cancer patients to Her-2 peptides. Anticancer Res 17:45–53
Giuliano AE, Sparks FC, Patterson K, Spears I, Morton DL (1986) Adjuvant chemo-immunotherapy in stage II carcinoma of the breast. J Surg Oncol 31:255–259
Gückel B, Lindauer M, Rudy W, Habicht A, Siebels M, Kaul S, Bastert G, Meuer SC, Moebius U (1995) CD80-transfected human breast and ovarian tumor cell lines: improved immunogenicity and induction of cytolytic CD8+ T lymphocytes. Cytokines Mol Ther 1:211–221
Gückel B, Meyer GC, Rudy W, Batrla R, Meuer SC, Bastert G, Wallwiener D, Moebius U (1999) Interleukin-12 requires initial CD80-mediated T-cell activation to support immune responses toward human breast and ovarian carcinoma. Cancer Gene Ther 6:228–237
Gückel B, Meuer S, Bastert G, Wallwiener D (2003) Tumor-associated antigens as tools in immunodiagnostics and immunotherapy of breast cancer. Geburtsh Frauenheilk 64:130–139
Habicht A, Lindauer M, Galmbacher P, Rudy W, Gebert J, Schackert HK, Meuer SC, Moebius U (1995) Development of immunogenic colorectal cancer cell lines for vaccination: expression of CD80 (B7.1) is not sufficient to restore impaired primary T cell activation in vitro. Eur J Cancer 31A:2396–2402
Hellström KE, Hellström I, Chen L (1995) Can co-stimulated tumor immunity be therapeutically efficacious? Immunol Rev 145:123–145
Hicklin DJ, Marincola FM, Ferrone S (1999) HLA class I antigen downregulation in human cancers: T-cell immunotherapy revives an old story. Mol Med Today 5:178–186
Hirano N, Takahashi T, Takahashi T, Azuma M, Okumura K, Yazaki Y, Yagita H, Hirai H (1997) Protective and therapeutic immunity against leukemia induced by irradiated B7-1 (CD80)-transduced leukemic cells. Hum Gene Ther 8:1375–1384
Imro MA, Dellabona P, Manici S, Heltai S, Consogno G, Bellone M, Rugarli C, Protti MP (1998) Human melanoma cells transfected with the B7-2 co-stimulatory molecule induce tumor-specific CD8+ cytotoxic T lymphocytes in vitro. Hum Gene Ther 9:1335–1344
Jäger E, Jager D, Knuth A (1998) Strategies for the development of vaccines to treat breast cancer. Recent Results Cancer Res 152:94–102
Jiang XP, Yang DC, Elliott RL, Head JF (2000) Vaccination with a mixed vaccine of autogenous and allogeneic breast cancer cells and tumor associated antigens CA15-3, CEA and CA125–results in immune and clinical responses in breast cancer patients. Cancer Biother Radiopharm 15:495–505
Jung D, Jaeger E, Cayeux S, Blankenstein T, Hilmes C, Karbach J, Moebius U, Knuth A, Huber C, Seliger B (1998) Strong immunogenic potential of a B7 retroviral expression vector: generation of HLA-B7-restricted CTL response against selectable marker genes. Hum Gene Ther 9:53–62
Kammula US, Lee KH, Riker AI, Wang E, Ohnmacht GA, Rosenberg SA, Marincola FM (1999) Functional analysis of antigen-specific T lymphocytes by serial measurement of gene expression in peripheral blood mononuclear cells and tumor specimens. J Immunol 163:6867–6875
Kammula US, Marincola FM, Rosenberg SA (2000) Real-time quantitative polymerase chain reaction assessment of immune reactivity in melanoma patients after tumor peptide vaccination. J Natl Cancer Inst 92:1336–1344
Kaufmann AM, Gissmann L, Schreckenberger C, Qiao L (1997) Cervical carcinoma cells transfected with the CD80 gene elicit a primary cytotoxic T lymphocyte response specific for HPV 16 E7 antigens. Cancer Gene Ther 4:377–382
Kayser S, Watermann I, Rentzsch C, Weinschenk T, Wallwiener D, Gückel B (2003) Tumor-associated antigen profiling in breast and ovarian cancer: mRNA, protein or T cell recognition? J Cancer Res Clin Oncol 129:397–409
Knight BC, Souberbielle BE, Rizzardi GP, Ball SE, Dalgleish AG (1996) Allogeneic murine melanoma cell vaccine: a model for the development of human allogeneic cancer vaccine. Melanoma Res 6:299–306
Kruse N, Pette M, Toyka K, Rieckmann P (1997) Quantification of cytokine mRNA expression by RT PCR in samples of previously frozen blood. J Immunol Methods 210:195–203
Li Y, McGowan P, Hellstrom I, Hellstrom KE, Chen L (1994) Costimulation of tumor-reactive CD4+ and CD8+ T lymphocytes by B7, a natural ligand for CD28, can be used to treat established mouse melanoma. J Immunol 153:421–428
Lindauer M, Rudy W, Gückel B, Doeberitz MV, Meuer SC, Moebius U (1998) Gene transfer of costimulatory molecules into a human colorectal cancer cell line: requirement of CD54, CD80 and class II MHC expression for enhanced immunogenicity. Immunology 93:390–397
Lustgarten J, Theobald M, Labadie C, LaFace D, Peterson P, Disis ML, Cheever MA, Sherman LA (1997) Identification of Her-2/neu CTL epitopes using double transgenic mice expressing HLA-A2.1 and human CD8. Hum Immunol 52:109–118
Meuer SC, Gückel B, Lindauer M, Rudy W, Moebius U (1998) Construction of immunogenic tumor cell surfaces by somatic gene transfer. Recent Results Cancer Res 144:78–85
Meyer GC, Batrla R, Rudy W, Meuer SC, Wallwiener D, Gückel B, Moebius U (1999) Potential of CD80-transfected human breast carcinoma cells to induce peptide-specific T lymphocytes in an allogeneic human histocompatibility leukocyte antigens (HLA)-A2.1+-matched situation. Cancer Gene Ther 6:282–288
Osanto S, Schiphorst PP, Weijl NI, Dijkstra N, Van Wees A, Brouwenstein N, Vaessen N, Van Krieken JH, Hermans J, Cleton FJ, Schrier PI (2000) Vaccination of melanoma patients with an allogeneic, genetically modified interleukin 2-producing melanoma cell line. Hum Gene Ther 11:739–750
Pascolo S, Schirle M, Gückel B, Dumrese T, Stumm S, Kayser S, Moris A, Wallwiener D, Rammensee H-G, Stevanović S (2001) A MAGE-A1 HLA-A*0201 epitope identified by mass spectrometry. Cancer Res 61:4072–4077
Plautz GE, Yang ZY, Wu BY, Gao X, Huang L, Nabel GJ (1993) Immunotherapy of malignancy by in vivo gene transfer into tumors. Proc Natl Acad Sci U S A 90:4645–4649
Rammensee HG, Bachmann J, Emmerich NN, Bachor OA, Stevanovic S (1999) SYFPEITHI: database for MHC ligands and peptide motifs. Immunogenetics 50:213–219. http://www.uni-tuebingen.de/uni/kxi/
Rentzsch C, Kayser S, Stumm S, Watermann I, Walter S, Stevanoviæ S, Wallwiener D, Gückel B (2003) Evaluation of pre-existent immunity in patients with primary breast cancer: molecular and cellular assays to quantify antigen-specific T lymphocytes in peripheral blood mononuclear cells. Clin Cancer Res 9:4376–4386
Riker AI, Kammula US, Panelli MC, Wang E, Ohnmacht GA, Steinberg SM, Rosenberg SA, Marincola FM (2000) Threshold levels of gene expression of the melanoma antigen gp100 correlate with tumor cell recognition by cytotoxic T lymphocytes. Int J Cancer 86:818–826
Ruiz-Cabello F, Garrido F (1998) HLA and cancer: from research to clinical impact. Immunol Today 19:539–542
Salcedo M, Momburg F, Hämmerling GJ, Ljunggren HG (1994) Resistance to natural killer cell lysis conferred TAP1/2 genes in human antigen-processing mutant cells. J Immunol 152:1702–1708
Scardino A, Alves P, Gross DA, Tourdot S, Graff-Dubois S, Angevin E, Firat H, Chouaib S, Lemonnier F, Nadler LM, Cardoso AA, Kosmatopoulos K (2001) Identification of HER-2/neu immunogenic epitopes presented by renal cell carcinoma and other human epithelial tumors. Eur J Immunol 31:3261–3270
Schendel DJ, Frankenberger B, Jantzer P, Cayeux S, Nobetaner E, Willimsky G, Maget B, Pohla H, Blankenstein T (2000) Expression of B7.1 (CD80) in a renal cell carcinoma line allows expansion of tumor-associated cytotoxic T lymphocytes in the presence of an alloresponse. Gene Ther 7:2007–2014
Schirle M, Keilholz W, Weber B, Gouttefangeas C, Dumrese T, Becker HD, Stevanovic S, Rammensee HG (2000) Identification of tumor-associated MHC class I ligands by a novel T cell-independent approach. Eur J Immunol 30:2216–2225
Schmidt W, Buschle M, Zauner W, Kirlappos H, Mechtler K, Trska B, Birnstiel ML (1997) Cell-free tumor antigen peptide-based cancer vaccines. Proc Natl Acad Sci U S A 94:3262–3267
Stevens EJ, Jacknin L, Robbins PF, Kawakami Y, el Gamil M, Rosenberg SA, Yannelli JR (1995) Generation of tumor-specific CTLs from melanoma patients by using peripheral blood stimulated with allogeneic melanoma tumor cell lines: fine specificity and MART-1 melanoma antigen recognition. J Immunol 154:762–771
Sundquist M, Thorstenson S, Brudin L, Wingren S, Nordenskjold B (2002) Incidence and prognosis in early onset breast cancer. Breast 11:30–35
Toes RE, Blom RJ, van der Voort E, Offringa R, Melief CJ, Kast WM (1996) Protective antitumor immunity induced by immunization with completely allogeneic tumor cells. Cancer Res 56:3782–3787
Townsend SE, Allison JP (1993) Tumor rejection after direct costimulation of CD8+ T cells by B7-transfected melanoma cells. Science 259:368–370
Wang YC, Zhu L, McHugh R, Graham SD Jr, Hillyer CD, Dillehay D, Sell KW, Selvaraj P (1996) Induction of autologous tumor-specific cytotoxic T-lymphocyte activity against a human renal carcinoma cell line by B7-1 (CD80) costimulation. J Immunother Emphasis Tumor Immunol 19:1–8
Wiseman C, Jessup JM, Smith TL, Hersh E, Bowen J, Blumenshein G (1982) Inflammatory breast cancer treated with surgery, chemotherapy and allogeneic tumor cell/BCG immunotherapy. Cancer 49:1266–1271
Zaks TZ, Rosenberg SA (1998) Immunization with a peptide epitope (p369–377) from HER-2/neu leads to peptide-specific cytotoxic T lymphocytes that fail to recognize HER-2/neu+ tumors. Cancer 58:4902–4908
Acknowledgements
Parts of this research were supported by grants from the Angewandte Klinische Forschung (AKF)-programm of the Medizinische Fakultät, University of Tübingen, the Forschungskommission (FoKo) der Medizinische Fakultät, University of Heidelberg, and by the Bundesministerium für Bildung und Forschung (BMBF), Germany.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gückel, B., Stumm, S., Rentzsch, C. et al. A CD80-transfected human breast cancer cell variant induces HER-2/neu–specific T cells in HLA-A*02–matched situations in vitro as well as in vivo. Cancer Immunol Immunother 54, 129–140 (2005). https://doi.org/10.1007/s00262-004-0583-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-004-0583-z