Abstract
Purpose
To evaluate if machine learning (ML) of radiomic features extracted from apparent diffusion coefficient (ADC) and T2-weighted (T2W) MRI can predict prostate cancer (PCa) diagnosis in Prostate Imaging-Reporting and Data System (PI-RADS) version 2.1 category 3 lesions.
Methods
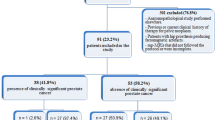
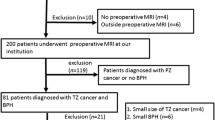
This multi-institutional review board-approved retrospective case–control study evaluated 158 men with 160 PI-RADS category 3 lesions (79 peripheral zone, 81 transition zone) diagnosed at 3-Tesla MRI with histopathology diagnosis by MRI-TRUS-guided targeted biopsy. A blinded radiologist confirmed PI-RADS v2.1 score and segmented lesions on axial T2W and ADC images using 3D Slicer, extracting radiomic features with an open-source software (Pyradiomics). Diagnostic accuracy for (1) any PCa and (2) clinically significant (CS; International Society of Urogenital Pathology Grade Group ≥ 2) PCa was assessed using XGBoost with tenfold cross -validation.
Results
From 160 PI-RADS 3 lesions, there were 50.0% (80/160) PCa, including 36.3% (29/80) CS-PCa (63.8% [51/80] ISUP 1, 23.8% [19/80] ISUP 2, 8.8% [7/80] ISUP 3, 3.8% [3/80] ISUP 4). The remaining 50.0% (80/160) lesions were benign. ML of all radiomic features from T2W and ADC achieved area under receiver operating characteristic curve (AUC) for diagnosis of (1) CS-PCa 0.547 (95% Confidence Intervals 0.510–0.584) for T2W and 0.684 (CI 0.652–0.715) for ADC and (2) any PCa 0.608 (CI 0.579–0.636) for T2W and 0.642 (CI 0.614–0.0.670) for ADC.
Conclusion
Our results indicate ML of radiomic features extracted from T2W and ADC achieved at best moderate accuracy for determining which PI-RADS category 3 lesions represent PCa.







Similar content being viewed by others
References
Turkbey B, Rosenkrantz AB, Haider MA et al (2019) Prostate Imaging Reporting and Data System Version 2.1: 2019 Update of Prostate Imaging Reporting and Data System Version 2. Eur Urol 76:340-351
Schieda N, Lim CS, Zabihollahy F et al (2021) Quantitative Prostate MRI. Journal of Magnetic Resonance Imaging 53:1632-1645
Purysko AS, Baroni RH, Giganti F et al (2020) PI-RADS Version 2.1: A Critical Review, From the AJR Special Series on Radiology Reporting and Data Systems. American Journal of Roentgenology 216:20-32
Westphalen AC, McCulloch CE, Anaokar JM et al (2020) Variability of the Positive Predictive Value of PI-RADS for Prostate MRI across 26 Centers: Experience of the Society of Abdominal Radiology Prostate Cancer Disease-focused Panel. Radiology 296:76-84
Barkovich EJ, Shankar PR, Westphalen AC (2019) A Systematic Review of the Existing Prostate Imaging Reporting and Data System Version 2 (PI-RADSv2) Literature and Subset Meta-Analysis of PI-RADSv2 Categories Stratified by Gleason Scores. AJR Am J Roentgenol 212:847-854
Zhang L, Tang M, Chen S, Lei X, Zhang X, Huan Y (2017) A meta-analysis of use of Prostate Imaging Reporting and Data System Version 2 (PI-RADS V2) with multiparametric MR imaging for the detection of prostate cancer. Eur Radiol 27:5204-5214
Rudolph MM, Baur ADJ, Cash H et al (2020) Diagnostic performance of PI-RADS version 2.1 compared to version 2.0 for detection of peripheral and transition zone prostate cancer. Sci Rep 10:15982
Otti VC, Miller C, Powell RJ, Thomas RM, McGrath JS (2019) The diagnostic accuracy of multiparametric magnetic resonance imaging before biopsy in the detection of prostate cancer. BJU Int 123:82-90
Schoots IG (2018) MRI in early prostate cancer detection: how to manage indeterminate or equivocal PI-RADS 3 lesions? Transl Androl Urol 7:70-82
Maggi M, Panebianco V, Mosca A et al (2020) Prostate Imaging Reporting and Data System 3 Category Cases at Multiparametric Magnetic Resonance for Prostate Cancer: A Systematic Review and Meta-analysis. Eur Urol Focus 6:463-478
Padhani AR, Barentsz J, Villeirs G et al (2019) PI-RADS Steering Committee: The PI-RADS Multiparametric MRI and MRI-directed Biopsy Pathway. Radiology 292:464-474
Felker ER, Raman SS, Margolis DJ et al (2017) Risk Stratification Among Men With Prostate Imaging Reporting and Data System version 2 Category 3 Transition Zone Lesions: Is Biopsy Always Necessary? AJR Am J Roentgenol 209:1272-1277
Sheridan AD, Nath SK, Syed JS et al (2018) Risk of Clinically Significant Prostate Cancer Associated With Prostate Imaging Reporting and Data System Category 3 (Equivocal) Lesions Identified on Multiparametric Prostate MRI. AJR Am J Roentgenol 210:347-357
Washino S, Okochi T, Saito K et al (2017) Combination of prostate imaging reporting and data system (PI-RADS) score and prostate-specific antigen (PSA) density predicts biopsy outcome in prostate biopsy naive patients. BJU Int 119:225-233
Ullrich T, Quentin M, Arsov C et al (2018) Risk Stratification of Equivocal Lesions on Multiparametric Magnetic Resonance Imaging of the Prostate. J Urol 199:691-698
Hermie I, Van Besien J, De Visschere P, Lumen N, Decaestecker K (2019) Which clinical and radiological characteristics can predict clinically significant prostate cancer in PI-RADS 3 lesions? A retrospective study in a high-volume academic center. Eur J Radiol 114:92-98
Görtz M, Radtke JP, Hatiboglu G et al (2021) The Value of Prostate-specific Antigen Density for Prostate Imaging-Reporting and Data System 3 Lesions on Multiparametric Magnetic Resonance Imaging: A Strategy to Avoid Unnecessary Prostate Biopsies. Eur Urol Focus 7:325-331
Kan Y, Zhang Q, Hao J et al (2020) Clinico-radiological characteristic-based machine learning in reducing unnecessary prostate biopsies of PI-RADS 3 lesions with dual validation. Eur Radiol 30:6274-6284
Bonekamp D, Kohl S, Wiesenfarth M et al (2018) Radiomic Machine Learning for Characterization of Prostate Lesions with MRI: Comparison to ADC Values. Radiology 289:128-137
Chaddad A, Kucharczyk MJ, Cheddad A et al (2021) Magnetic Resonance Imaging Based Radiomic Models of Prostate Cancer: A Narrative Review. Cancers (Basel) 13
Woźnicki P, Westhoff N, Huber T et al (2020) Multiparametric MRI for Prostate Cancer Characterization: Combined Use of Radiomics Model with PI-RADS and Clinical Parameters. Cancers (Basel) 12
Giambelluca D, Cannella R, Vernuccio F et al (2021) PI-RADS 3 Lesions: Role of Prostate MRI Texture Analysis in the Identification of Prostate Cancer. Curr Probl Diagn Radiol 50:175-185
Brancato V, Aiello M, Basso L et al (2021) Evaluation of a multiparametric MRI radiomic-based approach for stratification of equivocal PI-RADS 3 and upgraded PI-RADS 4 prostatic lesions. Sci Rep 11:643
Wibmer A, Hricak H, Gondo T et al (2015) Haralick texture analysis of prostate MRI: utility for differentiating non-cancerous prostate from prostate cancer and differentiating prostate cancers with different Gleason scores. Eur Radiol. https://doi.org/10.1007/s00330-015-3701-8
Vignati A, Mazzetti S, Giannini V et al (2015) Texture features on T2-weighted magnetic resonance imaging: new potential biomarkers for prostate cancer aggressiveness. Phys Med Biol 60:2685-2701
Stanzione A, Cuocolo R, Cocozza S et al (2019) Detection of Extraprostatic Extension of Cancer on Biparametric MRI Combining Texture Analysis and Machine Learning: Preliminary Results. Acad Radiol 26:1338-1344
Lim C, Flood TA, Hakim SW et al (2016) Evaluation of apparent diffusion coefficient and MR volumetry as independent associative factors for extra-prostatic extension (EPE) in prostatic carcinoma. J Magn Reson Imaging 43:726-736
Schwier M, van Griethuysen J, Vangel MG et al (2019) Repeatability of Multiparametric Prostate MRI Radiomics Features. Sci Rep 9:9441
Hou Y, Bao ML, Wu CJ, Zhang J, Zhang YD, Shi HB (2020) A radiomics machine learning-based redefining score robustly identifies clinically significant prostate cancer in equivocal PI-RADS score 3 lesions. Abdom Radiol (NY) 45:4223-4234
Hectors SJ, Chen C, Chen J et al (2021) Magnetic Resonance Imaging Radiomics-Based Machine Learning Prediction of Clinically Significant Prostate Cancer in Equivocal PI-RADS 3 Lesions. J Magn Reson Imaging. https://doi.org/10.1002/jmri.27692
Lim C, Abreu-Gomez J, Leblond MA et al (2020) When to biopsy Prostate Imaging and Data Reporting System version 2 (PI-RADSv2) assessment category 3 lesions? Use of clinical and imaging variables to predict cancer diagnosis at targeted biopsy. Can Urol Assoc J.https://doi.org/10.5489/cuaj.6781
Abreu-Gomez J, Lim C, Cron GO, Krishna S, Sadoughi N, Schieda N (2021) Pharmacokinetic modeling of dynamic contrast-enhanced (DCE)-MRI in PI-RADS category 3 peripheral zone lesions: preliminary study evaluating DCE-MRI as an imaging biomarker for detection of clinically significant prostate cancers. Abdom Radiol (NY). https://doi.org/10.1007/s00261-021-03035-6
van Griethuysen JJM, Fedorov A, Parmar C et al (2017) Computational Radiomics System to Decode the Radiographic Phenotype. Cancer Res 77:e104-e107
Chen T, Guestrin C (2016) XGBoost: A Scalable Tree Boosting System
Schieda N, Nguyen K, Thornhill RE, McInnes MDF, Wu M, James N (2020) Importance of phase enhancement for machine learning classification of solid renal masses using texture analysis features at multi-phasic CT. Abdom Radiol (NY) 45:2786–2796
Hodgdon T, Thornhill RE, James ND, Beaulé PE, Speirs AD, Rakhra KS (2020) CT texture analysis of acetabular subchondral bone can discriminate between normal and cam-positive hips. Eur Radiol 30:4695–4704
authors TG (2016) GPyOpt: A Bayesian Optimization framework in Python.
Thornton C, Hutter F, Hoos HH, Leyton-Brown K Auto-WEKA: Combined selection and hyperparameter optimization of classification algorithms. ACM, pp 847–855
Kohavi R (1995) A study of cross-validation and bootstrap for accuracy estimation and model selectionProceedings of the 14th international joint conference on Artificial intelligence - Volume 2. Morgan Kaufmann Publishers Inc., Montreal, Quebec, Canada, pp 1137–1143
Rosenkrantz AB, Meng X, Ream JM et al (2016) Likert score 3 prostate lesions: Association between whole-lesion ADC metrics and pathologic findings at MRI/ultrasound fusion targeted biopsy. J Magn Reson Imaging 43:325-332
Schelb P, Kohl S, Radtke JP et al (2019) Classification of Cancer at Prostate MRI: Deep Learning versus Clinical PI-RADS Assessment. Radiology 293:607-617
Varghese B, Chen F, Hwang D et al (2019) Objective risk stratification of prostate cancer using machine learning and radiomics applied to multiparametric magnetic resonance images. Sci Rep 9:1570
Zhong X, Cao R, Shakeri S et al (2019) Deep transfer learning-based prostate cancer classification using 3 Tesla multi-parametric MRI. Abdom Radiol (NY) 44:2030-2039
Li J, Weng Z, Xu H et al (2018) Support Vector Machines (SVM) classification of prostate cancer Gleason score in central gland using multiparametric magnetic resonance images: A cross-validated study. Eur J Radiol 98:61-67
Li M, Yang L, Yue Y, Xu J, Huang C, Song B (2021) Use of Radiomics to Improve Diagnostic Performance of PI-RADS v2.1 in Prostate Cancer. Frontiers in Oncology 10
Nketiah GA, Elschot M, Scheenen TW et al (2021) Utility of T2-weighted MRI texture analysis in assessment of peripheral zone prostate cancer aggressiveness: a single-arm, multicenter study. Scientific Reports 11:2085
Litjens GJ, Hambrock T, Hulsbergen-van de Kaa C, Barentsz JO, Huisman HJ (2012) Interpatient variation in normal peripheral zone apparent diffusion coefficient: effect on the prediction of prostate cancer aggressiveness. Radiology 265:260-266
Schmeel FC (2019) Variability in quantitative diffusion-weighted MR imaging (DWI) across different scanners and imaging sites: is there a potential consensus that can help reducing the limits of expected bias? Eur Radiol 29:2243-2245
Barrett T, Lawrence EM, Priest AN et al (2019) Repeatability of diffusion-weighted MRI of the prostate using whole lesion ADC values, skew and histogram analysis. Eur J Radiol 110:22-29
Balakrishnan AS, Cowan JE, Cooperberg MR, Shinohara K, Nguyen HG, Carroll PR (2019) Evaluating the Safety of Active Surveillance: Outcomes of Deferred Radical Prostatectomy after an Initial Period of Surveillance. J Urol 202:506-510
Schieda N, Lim CS, Zabihollahy F et al (2020) Quantitative Prostate MRI. J Magn Reson Imaging. https://doi.org/10.1002/jmri.27191
Surov A, Meyer HJ, Wienke A (2019) Correlations between Apparent Diffusion Coefficient and Gleason Score in Prostate Cancer: A Systematic Review. Eur Urol Oncol. https://doi.org/10.1016/j.euo.2018.12.006
Wegelin O, Exterkate L, van der Leest M et al (2019) The FUTURE Trial: A Multicenter Randomised Controlled Trial on Target Biopsy Techniques Based on Magnetic Resonance Imaging in the Diagnosis of Prostate Cancer in Patients with Prior Negative Biopsies. Eur Urol 75:582-590
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Christopher S. Lim has cooperative research development agreements with IBM Watson Health Imaging. All other authors have no relevant disclosures.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendices
Appendix 1: MRI protocols for both institution
Institution 1 techniquea,b | Imaging plane | Field of view (mm) | Matrix size | Slice thickness/gap (mm) | TR/TE (ms) | Echo train length | Flip angle | Acceleration factor | Receiver bandwidth (Hz/voxel) | Acquisition time (min) | Number of signals averaged |
|---|---|---|---|---|---|---|---|---|---|---|---|
T2 TSE | Coronal Sagittal Axial | 220 × 220 | 320 × 256 | 4.0/0 3.0/0 3.0/0 | 3890–5250/ 105–125 | 27–35 | 111 | N/A | 122 | 4 min 4 min 4 min | 1–2 |
DWIe | Axial | 280 × 280 | 128 × 80 | 3–5.0/0 | 4200/ 90 | 1 | 90 | 2 | 1950 | 5 min | 4–10 |
T1 GREf dynamic contrast | Axial | 220 × 220 | 128 × 128 | 4.0/0 | 4.3/1.3 | N/A | 12 | 2 | 488 | 2 min | 1 |
Institution 2 techniquea,b | Imaging plane | Field of view (mm) | Matrix size | Slice thickness/gap (mm) | TR/TE (ms) | Echo train length | Flip angle | Acceleration factor | Receiver bandwidth (Hz/voxel) | Approximate acquisition time (min) | Number of signals averaged |
|---|---|---|---|---|---|---|---|---|---|---|---|
T2 TSEc | Coronal Sagittal Axial | 220 × 220 | 320 × 256 | 3.0/0 3.0/0 3.0/0 | 3890–5250/ 105–125 | 27–35 | 111 | N/A | 122 | 4 min 4 min 4 min | 1–2 |
DWId | Axial | 220 × 220 | 128 × 80 | 3.0/0 | 4200/ 90 | 1 | 90 | 2 | 1950 | 5 min | 4–15 |
T1 GREf dynamic contrast | Axial | 220 × 220 | 128 × 128 | 3.0/0 | 4.3/1.3 | N/A | 12 | 2 | 488 | 5 min | 1 |
Appendix 2: Cognitive fusion biopsy technique used at both institutions
Targeted biopsies were performed using TRUS guidance with cognitive fusion of MRI data onto real time 2-Dimensional TRUS images. At institution 1, transrectal US-guided examinations were performed using modern Ultrasound equipment (Aloka Prosound Alpha 10, Aloka Hitachi Medical or General Electric Logiq E9, General Electric Healthcare) and endoluminal 4–8 MHz end-fire probes. Biopsies were performed by a core-group of five fellowship-trained abdominal radiologists all with 6 years of experience in cognitive fusion-targeted biopsy of the prostate. At institution 2, transrectal US-guided examinations were performed using one system (Phillips IU 22, Philips medical) with endocavitary 5–9 MHz end-fire probes. Biopsies were performed by a core-group of three fellowship-trained abdominal radiologists with mean of 8.3 years of experience [range 3–15 years] in cognitive fusion-targeted biopsy of the prostate.
The TRUS-guided biopsy system used for all biopsies employed an 18-gauge side-cutting needle. All of the biopsy suites are equipped with monitors which enable display of mp-MRI which can be reviewed before and during the biopsy procedure. Anesthesia is provided using 1–2% Lidocaine nerve block. A fleet enema was prescribed prior to the procedure and antibiotic prophylaxis to prevent infection. Core-needle biopsy specimens are submitted for laboratory processing and interpretation in separate pathology specimen containers according to the site of sampling. Tissues from biopsy specimens are fixed overnight in 10% neutral buffered formalin. Three histological slides are prepared from each block, each with three serial sections cut at 3 μm in thickness and stained with hematoxylin and eosin (H&E). Biopsy results are reported for each core specimen individually.
Appendix 3: Accuracy and area under receiver operating characteristic curve (AUC) for the diagnosis of CS-PCa and any cancer for all features and the top 5 T2W and ADC least interdependent features in the combined and institution 2 data
Combined data | Institution 2 data | |||||
|---|---|---|---|---|---|---|
Accuracy (%) | AUC (95% confidence interval) | Accuracy (%) | AUC (95% confidence interval) | |||
T2W | CSPCa | All | 81.9 | 0.547 (0.510–0.584) | 83.9 | 0.550 (0.507–0.591) |
Top 5 | 81.9 | 0.594 (0.560–0.628) | 83.9 | 0.607 (0.571–0.643) | ||
Any cancer | All | 60.1 | 0.608 (0.579–0.636) | 53.6 | 0.535 (0.462–0.608) | |
Top 5 | 61.8 | 0.632 (0.605–0.660) | 54.8 | 0.608 (0.537–0.680) | ||
ADC | CSPCa | All | 81.4 | 0.684 (0.652–0.715) | 82.3 | 0.715 (0.677–0.753) |
Top 5 | 81.9 | 0.620 (0.585–0.654) | 83.9 | 0.605 (0.565–0.645) | ||
Any cancer | All | 60.6 | 0.642 (0.614–0.670) | 62.5 | 0.660 (0.590–0.728) | |
Top 5 | 66.3 | 0.695 (0.668–0.720) | 68.2 | 0.725 (0.659–0.791) | ||
Top 5 T2W and ADC | CSPCa | 81.9 | 0.595 (0.560–0.630) | 83.9 | 0.634 (0.599–0.669) | |
Any cancer | 61.7 | 0.682 (0.655–0.707) | 64.1 | 0.694 (0.626–0.757) | ||
Rights and permissions
About this article
Cite this article
Lim, C.S., Abreu-Gomez, J., Thornhill, R. et al. Utility of machine learning of apparent diffusion coefficient (ADC) and T2-weighted (T2W) radiomic features in PI-RADS version 2.1 category 3 lesions to predict prostate cancer diagnosis. Abdom Radiol 46, 5647–5658 (2021). https://doi.org/10.1007/s00261-021-03235-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-021-03235-0