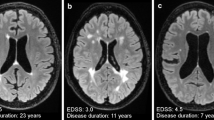
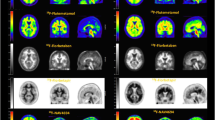
Abstract
Purpose
MRI-negative children with focal cortical dysplasia type II (FCD II) are one of the most challenging cases in surgical epilepsy management. We aimed to utilize quantitative positron emission tomography (QPET) analysis to complement [18F]SynVesT-1 and [18F]FDG PET imaging and facilitate the localization of epileptogenic foci in pediatric MRI-negative FCD II patients.
Methods
We prospectively enrolled 17 MRI-negative children with FCD II who underwent [18F]SynVesT-1 and [18F]FDG PET before surgical resection. The QPET scans were analyzed using statistical parametric mapping (SPM) with respect to healthy controls. The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and area under the curve (AUC) of [18F]SynVesT-1 PET, [18F]FDG PET, [18F]SynVesT-1 QPET, and [18F]FDG QPET in the localization of epileptogenic foci were assessed. Additionally, we developed a multivariate prediction model based on dual trace PET/QPET assessment.
Results
The AUC values of [18F]FDG PET and [18F]SynVesT-1 PET were 0.861 (sensitivity = 94.1%, specificity = 78.2%, PPV = 38.1%, NPV = 98.9%) and 0.908 (sensitivity = 82.4%, specificity = 99.2%, PPV = 93.3%, NPV = 97.5%), respectively. [18F]FDG QPET showed lower sensitivity (76.5%) and NPV (96.6%) but higher specificity (95.0%) and PPV (68.4%) than visual assessment, while [18F]SynVesT-1 QPET exhibited higher sensitivity (94.1%) and NPV (99.1%) but lower specificity (97.5%) and PPV (84.2%). The multivariate prediction model had the highest AUC value (AUC = 0.996, sensitivity = 100.0%, specificity = 96.6%, PPV = 81.0%, NPV = 100%).
Conclusions
The multivariate prediction model based on [18F]SynVesT-1 and [18F]FDG PET/QPET assessments holds promise in noninvasively identifying epileptogenic regions in MRI-negative children with FCD II. Furthermore, the combination of visual assessment and QPET may improve the sensitivity and specificity of diagnostic tests in localizing epileptogenic foci and achieving a preferable surgical outcome in MRI-negative FCD II.



Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
References
Duchowny M, Jayakar P, Resnick T, Harvey AS, Alvarez L, Dean P, et al. Epilepsy surgery in the first three years of life. Epilepsia. 1998;39:737–43. https://doi.org/10.1111/j.1528-1157.1998.tb01159.x
Blumcke I, Spreafico R, Haaker G, Coras R, Kobow K, Bien CG, et al. Histopathological findings in brain tissue obtained during Epilepsy surgery. N Engl J Med. 2017;377:1648–56. https://doi.org/10.1056/NEJMoa1703784
Harvey AS, Cross JH, Shinnar S, Mathern GW. Defining the spectrum of international practice in pediatric epilepsy surgery patients. Epilepsia. 2008;49:146–55. https://doi.org/10.1111/j.1528-1167.2007.01421.x
Chapman K, Wyllie E, Najm I, Ruggieri P, Bingaman W, Lüders J, et al. Seizure outcome after epilepsy surgery in patients with normal preoperative MRI. J Neurol Neurosurg Psychiatry. 2005;76:710–3. https://doi.org/10.1136/jnnp.2003.026757
Lee SK, Lee SY, Kim KK, Hong KS, Lee DS, Chung CK. Surgical outcome and prognostic factors of cryptogenic neocortical epilepsy. Ann Neurol. 2005;58:525–32. https://doi.org/10.1002/ana.20569
Colombo N, Tassi L, Galli C, Citterio A, Lo Russo G, Scialfa G, et al. Focal cortical dysplasias: MR imaging, histopathologic, and clinical correlations in surgically treated patients with epilepsy. AJNR Am J Neuroradiol. 2003;24:724–33.
Krsek P, Maton B, Korman B, Pacheco-Jacome E, Jayakar P, Dunoyer C, et al. Different features of histopathological subtypes of pediatric focal cortical dysplasia. Ann Neurol. 2008;63:758–69. https://doi.org/10.1002/ana.21398
Zhu Y, Feng J, Wu S, Hou H, Ji J, Zhang K, et al. Glucose Metabolic Profile by Visual Assessment combined with Statistical Parametric Mapping Analysis in Pediatric patients with Epilepsy. J Nucl Med. 2017;58:1293–9. https://doi.org/10.2967/jnumed.116.187492
von Oertzen TJ. PET and ictal SPECT can be helpful for localizing epileptic foci. Curr Opin Neurol. 2018;31:184–91. https://doi.org/10.1097/wco.0000000000000527
Liu F, Ruan W, Deng X, Song Y, Song W, Hu F, et al. Efficacy of delayed (18)F-FDG hybrid PET/MRI for epileptic focus identification: a prospective cohort study. Eur J Nucl Med Mol Imaging. 2021;48:293–301. https://doi.org/10.1007/s00259-020-04935-3
Kumar A, Juhasz C, Asano E, Sood S, Muzik O, Chugani HT. Objective detection of epileptic foci by 18F-FDG PET in children undergoing epilepsy surgery. J Nucl Med. 2010;51:1901–7. https://doi.org/10.2967/jnumed.110.075390
Chassoux F, Rodrigo S, Semah F, Beuvon F, Landre E, Devaux B, et al. FDG-PET improves surgical outcome in negative MRI Taylor-type focal cortical dysplasias. Neurology. 2010;75:2168–75. https://doi.org/10.1212/WNL.0b013e31820203a9
Tang Y, Yu J, Zhou M, Li J, Long T, Li Y, et al. Cortical abnormalities of synaptic vesicle protein 2A in focal cortical dysplasia type II identified in vivo with (18)F-SynVesT-1 positron emission tomography imaging. Eur J Nucl Med Mol Imaging. 2022;49:3482–91. https://doi.org/10.1007/s00259-021-05665-w
Mayoral M, Marti-Fuster B, Carreño M, Carrasco JL, Bargalló N, Donaire A, et al. Seizure-onset zone localization by statistical parametric mapping in visually normal (18) F-FDG PET studies. Epilepsia. 2016;57:1236–44. https://doi.org/10.1111/epi.13427
Guo K, Wang J, Wang Z, Wang Y, Cui B, Zhao G, et al. Morphometric analysis program and quantitative positron emission tomography in presurgical localization in MRI-negative epilepsies: a simultaneous PET/MRI study. Eur J Nucl Med Mol Imaging. 2022;49:1930–8. https://doi.org/10.1007/s00259-021-05657-w
Lin Y, Fang YD, Wu G, Jones SE, Prayson RA, Moosa ANV, et al. Quantitative positron emission tomography-guided magnetic resonance imaging postprocessing in magnetic resonance imaging-negative epilepsies. Epilepsia. 2018;59:1583–94. https://doi.org/10.1111/epi.14474
Kwan P, Arzimanoglou A, Berg AT, Brodie MJ, Allen Hauser W, Mathern G, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on therapeutic strategies. Epilepsia. 2010;51:1069–77. https://doi.org/10.1111/j.1528-1167.2009.02397.x
Blümcke I, Thom M, Aronica E, Armstrong DD, Vinters HV, Palmini A, et al. The clinicopathologic spectrum of focal cortical dysplasias: a consensus classification proposed by an ad hoc Task Force of the ILAE Diagnostic Methods Commission. Epilepsia. 2011;52:158–74. https://doi.org/10.1111/j.1528-1167.2010.02777.x
Li S, Cai Z, Wu X, Holden D, Pracitto R, Kapinos M, et al. Synthesis and in vivo evaluation of a novel PET Radiotracer for imaging of synaptic vesicle glycoprotein 2A (SV2A) in Nonhuman Primates. ACS Chem Neurosci. 2019;10:1544–54. https://doi.org/10.1021/acschemneuro.8b00526
Guedj E, Varrone A, Boellaard R, Albert NL, Barthel H, van Berckel B, et al. EANM procedure guidelines for brain PET imaging using [(18)F]FDG, version 3. Eur J Nucl Med Mol Imaging. 2022;49:632–51. https://doi.org/10.1007/s00259-021-05603-w
Li Y, Feng J, Zhang T, Shi K, Ding Y, Zhang X, et al. Brain metabolic characteristics distinguishing typical and atypical benign epilepsy with centro-temporal spikes. Eur Radiol. 2021;31:9335–45. https://doi.org/10.1007/s00330-021-08051-0
Therriault J, Pascoal TA, Savard M, Benedet AL, Chamoun M, Tissot C, et al. Topographic distribution of Amyloid-β, Tau, and atrophy in patients with Behavioral/Dysexecutive Alzheimer Disease. Neurology. 2021;96:e81–e92. https://doi.org/10.1212/wnl.0000000000011081
Pascoal TA, Benedet AL, Tudorascu DL, Therriault J, Mathotaarachchi S, Savard M, et al. Longitudinal 18F-MK-6240 tau tangles accumulation follows Braak stages. Brain. 2021;144:3517–28. https://doi.org/10.1093/brain/awab248
López-González FJ, Silva-Rodríguez J, Paredes-Pacheco J, Niñerola-Baizán A, Efthimiou N, Martín-Martín C, et al. Intensity normalization methods in brain FDG-PET quantification. NeuroImage. 2020;222:117229. https://doi.org/10.1016/j.neuroimage.2020.117229
Salzberg M, Taher T, Davie M, Carne R, Hicks RJ, Cook M, et al. Depression in temporal lobe epilepsy surgery patients: an FDG-PET study. Epilepsia. 2006;47:2125–30. https://doi.org/10.1111/j.1528-1167.2006.00860.x
Gill RS, Lee HM, Caldairou B, Hong SJ, Barba C, Deleo F, et al. Multicenter Validation of a deep learning detection algorithm for focal cortical dysplasia. Neurology. 2021;97:e1571–e82. https://doi.org/10.1212/wnl.0000000000012698
Hong SJ, Kim H, Schrader D, Bernasconi N, Bernhardt BC, Bernasconi A. Automated detection of cortical dysplasia type II in MRI-negative epilepsy. Neurology. 2014;83:48–55. https://doi.org/10.1212/wnl.0000000000000543
Kim YH, Kang HC, Kim DS, Kim SH, Shim KW, Kim HD, et al. Neuroimaging in identifying focal cortical dysplasia and prognostic factors in pediatric and adolescent epilepsy surgery. Epilepsia. 2011;52:722–7. https://doi.org/10.1111/j.1528-1167.2010.02950.x
Wong CH, Bleasel A, Wen L, Eberl S, Byth K, Fulham M, et al. The topography and significance of extratemporal hypometabolism in refractory mesial temporal lobe epilepsy examined by FDG-PET. Epilepsia. 2010;51:1365–73. https://doi.org/10.1111/j.1528-1167.2010.02552.x
Li S, Naganawa M, Pracitto R, Najafzadeh S, Holden D, Henry S, et al. Assessment of test-retest reproducibility of [(18)F]SynVesT-1, a novel radiotracer for PET imaging of synaptic vesicle glycoprotein 2A. Eur J Nucl Med Mol Imaging. 2021;48:1327–38. https://doi.org/10.1007/s00259-020-05149-3
Naganawa M, Li S, Nabulsi N, Henry S, Zheng MQ, Pracitto R, et al. First-in-human evaluation of (18)F-SynVesT-1, a Radioligand for PET imaging of synaptic vesicle glycoprotein 2A. J Nucl Med. 2021;62:561–7. https://doi.org/10.2967/jnumed.120.249144
Xiao L, Zhou M, Tang Y, Hu S. (18) F-SynVesT-1 positron emission tomography in a hypothalamic hamartoma with abnormal uptake. Epilepsia. 2023;64:e43-e7. https://doi.org/10.1111/epi.17533
Toering ST, Boer K, de Groot M, Troost D, Heimans JJ, Spliet WG, et al. Expression patterns of synaptic vesicle protein 2A in focal cortical dysplasia and TSC-cortical tubers. Epilepsia. 2009;50:1409–18. https://doi.org/10.1111/j.1528-1167.2008.01955.x
Dangouloff-Ros V, Fillon L, Eisermann M, Losito E, Boisgontier J, Charpy S, et al. Preoperative Detection of Subtle Focal Cortical Dysplasia in children by combined arterial spin labeling, Voxel-based morphometry, Electroencephalography-synchronized functional MRI, resting-state Regional Homogeneity, and 18F-fluorodeoxyglucose Positron Emission Tomography. Neurosurgery. 2022. https://doi.org/10.1227/neu.0000000000002310
Ponisio MR, Zempel JM, Day BK, Eisenman LN, Miller-Thomas MM, Smyth MD, et al. The role of SPECT and PET in Epilepsy. AJR Am J Roentgenol. 2021;216:759–68. https://doi.org/10.2214/ajr.20.23336
Muzik O, Chugani DC, Juhász C, Shen C, Chugani HT. Statistical parametric mapping: assessment of application in children. NeuroImage. 2000;12:538–49. https://doi.org/10.1006/nimg.2000.0651
Acknowledgements
We extend our deepest appreciation to the research participants and their families. We are grateful to Professor Yiyun Huang from Yale University PET Center for providing the prodrug and synthesis method of [18F]SynVesT-1.
Declarations.
Funding
This study has received funding from the National Key Program of China (2022YFC2503804), the National Natural Science Foundation of China (82071461, 81801740, 91859207, 82271503, and 81771873), the National Science Foundation of Hunan Province (2020JJ5922, 2021JJ31060), Science and Technology Innovation Program of Hunan Province (2021RC4056), clinical research foundation of the National Clinical Research Center for Geriatric Diseases (XIANGYA) (2020LNJJ01), and China Postdoctoral Science Foundation (2022M23561).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Ling Xiao, Jinhui Yang, Yongxiang Tang, Li Feng, Haoyue Zhu, Ming Zhou and Jian Li. Dingyang Liu performed the epilepsy surgery and provided prognostic information. The first draft of the manuscript was written by Ling Xiao and Jinhui Yang. Yongxiang Tang, Li Feng, and Shuo Hu revised the work critically for important intellectual content. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have declared that no competing interest exists.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Xiangya Hospital, Central South University.
Consent for participate
Informed consent was obtained from all individual participants or legal guardians included in the study.
Consent for publish
Patients signed informed consent regarding publishing their data and photograph.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xiao, L., Yang, J., Zhu, H. et al. [18F]SynVesT-1 and [18F]FDG quantitative PET imaging in the presurgical evaluation of MRI-negative children with focal cortical dysplasia type II. Eur J Nucl Med Mol Imaging 51, 1651–1661 (2024). https://doi.org/10.1007/s00259-024-06593-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-024-06593-1