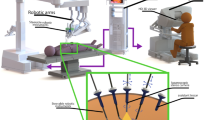
Abstract
Purpose
The recently introduced tethered DROP-IN gamma probe has revolutionized the way robotic radioguided surgery is performed, fully exploiting the nature of steerable robotic instruments. Given this success, the current first-in-human study investigates if the DROP-IN can also provide benefit in combination with steerable non-robotic instruments during conventional laparoscopic surgery, showing equivalence or even benefit over a traditional rigid gamma probe.
Methods
The evaluation was performed in ten patients during laparoscopic cervical (n = 4) and endometrial (n = 6) cancer sentinel lymph node (SLN) procedures. Surgical guidance was provided using the hybrid, or bi-modal, SLN tracer ICG-99mTc-nanocolloid. SLN detection was compared between the traditional rigid laparoscopic gamma probe, the combination of a DROP-IN gamma probe and a steerable laparoscopic instrument (LaproFlex), and fluorescence imaging.
Results
The gynecologists experienced an enlarged freedom of movement when using the DROP-IN + LaproFlex combination compared to the rigid laparoscopic probe, making it possible to better isolate the SLN signal from background signals. This did not translate into a change in the SLN find rate yet. In both cervical and endometrial cancer combined, the rigid probe and DROP-IN + LaproFlex combination provided an equivalent detection rate of 96%, while fluorescence provided 85%.
Conclusion
We have successfully demonstrated the in-human use of steerable DROP-IN radioguidance during laparoscopic cervical and endometrial cancer SLN procedures, expanding the utility beyond robotic procedures. Indicating an improved surgical experience, these findings encourage further investigation and consideration on a path towards routine clinical practice and improved patient outcome.
Trial registration
HCB/2021/0777 and NCT04492995; https://clinicaltrials.gov/study/NCT04492995
Similar content being viewed by others
Introduction
In the management of many different cancer types, information on lymph node (LN) involvement helps to determine the disease status (i.e., node-positive N1 vs. node-negative N0) and the selection of optimal treatment planning. Although non-invasive imaging is increasingly contributing in the diagnosis of macroscopic tumor spread (e.g., 18F-FDG PET in cervical cancer) [1], surgical LN dissection and subsequent histopathological examination still provides the most accurate method to identify the presence of LN micro-metastases [2, 3]. Acting on the premise that cancer metastases pass through a group of gatekeeper LNs (i.e., the first draining LNs from the primary tumor side called the sentinel LNs (SLNs)), the SLN procedure was introduced to help reduce surgical complications associated with extensive LN dissections [4], while optimally identifying tumor-related lymphatics [5]. This procedure focuses on retrieval and detailed pathological investigation of these SLNs, facilitating ultra-staging of the important LN samples and, at the same time, helping to reduce the workload at pathology [6, 7]. The SLN procedure has become routine in various forms of cancer (e.g., breast cancer [8], melanoma [9], penile cancer [10]) and is increasingly being extended to other fields as well (e.g., prostate cancer [11], cervical cancer [12], endometrial cancer [13], bladder cancer [14], gastric cancer [15]), implementations that allow for a distinguishment to be made between patients who have oncological benefit from (invasive) nodal treatment (e.g., extensive dissection, debulking, cytoreduction, or radiotherapy) and those who would not benefit.
To make it a patient-specific procedure, following the injection of a SLN-specific radiocolloid, preoperative lymphoscintigraphy and SPECT/CT imaging are used, imaging that helps to create a roadmap for surgical planning. Subsequent intraoperative localization of the identified targets is generally made possible using a gamma probe. This form of tracing delivers both in-depth audible and numerical feedback [16, 17]. By using hybrid, or bi-modal, SLN tracers (e.g., indocyanine green (ICG)-99mTc-nanocolloid) [18], the radioguidance can be complemented with superficial visual fluorescence guidance. While initial SN work initially occurred during surgeries performed in an open surgery setting, technological advancements have supported implementation in a minimal-invasive laparoscopic surgery setting [19]. To allow for radioguidance in a laparoscopic setting, gamma probe manufacturers have had to adapt their probe designs, essentially elongating the gamma probe design and making it narrow enough to fit through, for example, 12–15 mm trocars [20]. Unfortunately, having the trocar as pivot point drastically limits the probe movement, thus reducing the degrees of freedom (DoF) from six for open surgery to four for laparoscopic surgery (see Fig. 1A). In robotic surgery, these challenges have been overcome following the introduction of tethered DROP-IN gamma probes [21]. These detectors are completely inserted in the abdomen through a trocar, where they can be picked up and manipulated with the (robotic) laparoscopic instruments [22]. Initial evaluations during robotic prostate cancer SLN procedures [23, 24] have indicated this improved maneuverability (6 DoF), which translated into an improved SLN detection rate compared to rigid laparoscopic gamma probes (100% vs 76%, respectively). The value of this DROP-IN probe concept was further underlined by the successful evaluation with different probe designs [21, 25,26,27] and in different robotic indications within urology (PSMA-targeted surgery (i.e., in primary [25, 28] and recurrent [29, 30] prostate cancer)) and gynecology (cervical cancer SLN surgery [31]).
Overview of laparoscopic gamma probe applications investigated in this study. A Traditional rigid laparoscopic gamma probe, displaying available movement and intraoperative application. B DROP-IN probe usage with steerable laparoscopic instruments (i.e., DROP-IN + LaproFlex combination), displaying increased availability of movement and intraoperative application
The success of the DROP-IN probe in combination with the robotic steerable instruments makes one wonder if the technology can also provide benefit in combination with steerable (non-robotic) laparoscopic instruments. During laparoscopic cervical and endometrial cancer SLN procedures, we set out to evaluate if the combination of a DROP-IN gamma probe and a LaproFlex steerable laparoscopic instrument provides equivalence or even benefit over a traditional rigid gamma probe (see Fig. 1).
Patients and methods
Patient population and preoperative sentinel node mapping
This was a prospective pilot study. Ten patients with early-stage cervical cancer (n = 4) or low-to-intermediate-risk endometrial cancer (n = 6) were included between March and November 2022. All patients were scheduled for laparoscopic dissection of the primary tumor (i.e., radical hysterectomy) and SLN assessment. The inclusion criterion for cervical cancer was tumor stage IA1 with lymphovascular invasion, IA2 or IB1 (according to the International Federation of Gynecology and Obstetrics; FIGO 2018). Inclusion criteria for endometrial cancer were histology-proven endometrioid adenocarcinoma, but no involvement of the cervical stroma, and a myometrial invasion < 50% suspected. For both cancer types, the exclusion criteria were contraindication for surgical treatment, history of surgery or radiotherapy to nodal areas, and suspected metastatic disease based on preoperative imaging or preoperative biopsy.
Preoperative SLN mapping was based on both lymphoscintigraphy and SPECT/CT imaging using ICG-99mTc-nanocolloid [18]. Hybrid tracer preparation and injection were performed as previously described for cervical [32] and endometrial [33] cancer. Due to logistic reasons, one patient received the radioactive-only tracer 99mTc-nanocolloid. Eventual image reconstructions were examined by two nuclear medicine specialists and discussed with the surgical team prior to surgery. Patient characteristics and SLN mapping data are shown in Table 1. The study was approved by the local ethical committee (sub-study from NCT04492995—endometrial cancer; HCB/2021/0777—DROP-IN use in gynecological cancers) and informed consent was obtained from all individual patients included.
Surgical hardware and surgical procedure
Prior to the resection of the primary cancer (i.e., radical hysterectomy), a SLN dissection was performed. Preoperative SPECT/CT images served as a surgical roadmap on the number and location of the SLNs [34]. Both cervical and endometrial cancer procedures used a four trocar transperitoneal approach, with the patient in a Trendelenburg position. One 11-mm trocar was used for the laparoscopic camera, one 12-mm trocar for the gamma probes, and two 5-mm trocars for the laparoscopic instruments. After setting up the surgical field, the procedures started with the SLN localization. For intraoperative detection, a rigid traditional laparoscopic gamma probe (partially enclosed with sterile draping; Navigator, USSC, Norwalk, CT, USA) and a re-sterilizable DROP-IN gamma probe (see Fig. 1B; Drop-In Probe CXS-OP-DP, Crystal Photonics, Berlin, Germany) were used. As a steerable laparoscopic instrument, a 6 DoF short-fenestrated forceps LaproFlex instrument was used (DEAM, Amsterdam, The Netherlands). To support the comparison, individual SLN localizations were pursued with both the rigid gamma probe and the DROP-IN + LaproFlex combination. For confirmation of the SLN location, laparoscopic fluorescence imaging was used with either a Karl Storz fluorescence camera (KARL STORZ, Tuttlingen, Germany) or a Pinpoint fluorescence camera (Stryker, Kalamazoo, MI, USA). After excision of the SLN sample, confirmatory ex vivo measurements were performed with both gamma probe and fluorescence camera. The number and location of all SLNs excised were carefully reported, as well as their radioactive (counts) and fluorescent status (yes/no). Following surgery, all SLN specimens were carefully investigated for the presence of (micro-)metastases at histopathology, as described before [32, 33, 35].
The ten surgical procedures were performed by a total of four gynecologists, experienced in laparoscopic surgery. At the end of every procedure, the operating surgeon graded their experience (i.e., ease of use and available maneuverability) of the rigid gamma probe and DROP-IN + LaproFlex combination with one overall grade, ranging from 1 to 5 (where 5 was the most favorable grade).
Results
Preoperative imaging
Patient characteristics are summarized in Table 1. Median injected activity of ICG-99mTc-nanocolloid was 111 MBq for both cervical and endometrial cancer. SPECT/CT imaging revealed SLNs in all patients (i.e., no non-visualizations; see Fig. 2). For cervical cancer, a total of eight SLNs were detected (median of two per patient; IQR 0.5) of which seven were also seen on lymphoscintigraphy, with bilateral drainage in 75% of the patients. For endometrial cancer, a total of 18 SLNs were detected (median of three per patient; IQR 2) of which 16 were also seen on lymphoscintigraphy, with bilateral drainage in 67% of the patients. Due to the non-standard resection locations in low-risk endometrial cancer, one SLN at the presacral region right and three SLNs at the para-aortic region were not surgically pursued, leaving 14 surgical targets.
Preoperative sentinel lymph node mapping using nuclear imaging. A SPECT/CT imaging in cervix cancer, showing the sentinel node locations on a surface render overview (left) and detailed axial slices (middle and right). B SPECT/CT imaging in endometrial cancer, showing the sentinel node locations on a surface render overview (left) and detailed axial slices (middle and right)
Intraoperative sentinel node localization
During surgery (see Fig. 3), the DROP-IN probe was picked up by the LaproFlex instrument, with additional support from the bedside assistant either via the probe cable or via one of the additional laparoscopic instruments (Fig. 3E). The bending flexure joint of the LaproFlex, and the 45° grip design of the DROP-IN, supported a scanning range of − 90 to + 90° around the shaft of the instrument (Fig. 1B) and yielded a probe maneuverability with 6 DoF. The maximum turning circle (meaning the minimal distance needed for a complete 180° turn with the instrument positioned in its most bend state) was 15 cm. The scanning range of the traditional rigid gamma probe was fixed to a single view of 0° in front of the instrument shaft and a probe maneuverability with 4 DoF (Fig. 1A).
During radioguidance in cervical cancer, SLNs were retrieved from the following anatomical locations: 10% external iliac right, 40% obturator right, 10% inter-iliac right, 10% parametrial right, and 30% obturator left. All SLN locations (100%) could be traced using either the DROP-IN + LaproFlex combination or the rigid gamma probe. However, only 70% could be detected using fluorescence (Fig. 4). During radioguidance in endometrial cancer, SLNs were retrieved from the following anatomical locations: 23% inter-iliac right, 8% iliac communis right, 8% obturator right, 15% obturator left, 8% external iliac left, 23% inter-iliac left, 8% iliac communis left, and 8% aortic bifurcation. Of all SLNs surgically pursued, 93% were successfully located with the DROP-IN + LaproFlex combination and the rigid gamma probe, failing to detect one SLN in these six patients. Unfortunately, due to logistic reasons, ICG usage was not available in this specific patient, meaning only ten SLNs were pursued with fluorescence guidance, of which 100% could be detected (Fig. 4). Overall, combining both clinical indications, this gave rise to a detection rate of 96% (DROP-IN + LaproFlex combination), 96% (rigid gamma probe), and 85% (fluorescence).
Learning curve
The gynecologists required some training to be able to pick up and control the DROP-IN with the LaproFlex instrument, while they already had vast experience with the rigid gamma probe. Nevertheless, on average they rated their experience with the DROP-IN + LaproFlex more positive than that with the rigid gamma probe (a score of 5 versus 4, respectively). One of the reasons for this was that the increased maneuverability of the DROP-IN + LaproFlex combination made it feasible to perform gamma tracing of the SLNs without picking up background signal from the injection side. No side effects or patient complications were observed due to the DROP-IN + LaproFlex combination in vivo.
Pathology and overall sensitivity
In general, only SLNs were retrieved that were found based on the image-guided read-out. From the ten SLNs retrieved in cervical cancer, none was found to be tumor-positive after histopathologic evaluation. From the 13 SLNs retrieved in endometrial cancer, there was one tumor-positive SLN. As such, from a clinical outcome perspective and looking at a per-patient basis, the SLN procedure itself delivered a sensitivity and negative predictive value of both 100% in this study.
Discussion
The results from this study indicate that there is equivalence between the SLN detection achieved with a rigid laparoscopic gamma probe and the use of a DROP-IN probe with a steerable laparoscopic LaproFlex instrument. Hereby, this first-in-human evaluation (n = 10) was able to achieve the same accuracy as the rigid technology that the involved surgeons routinely apply for laparoscopic SLN procedures (two gynecologists > 10 years and two gynecologists > 20 years of experience). Unique to the DROP-IN + LaproFlex setup is that it provides increased flexibility and maneuverability in (non-robotic) laparoscopic surgery, a concept that is especially interesting for centers that do not have a robotic surgery program established. Moreover, we show the DROP-IN technology supports cervical and endometrial cancer SLN surgery, thereby broadening its indications.
The gynecologists experienced an enlarged freedom of movement when using the DROP-IN + LaproFlex combination compared to the rigid laparoscopic gamma probe. This feature helped to make it possible to better isolate the SLN signal from background signals (i.e., the injection side), yielding equivalent performance for this novel tracing approach: overall, both the DROP-IN and rigid probe achieved a 96% detection rate. While achieving equivalence to a well-established technology at an early stage of clinical implementation (n = 10) can be considered a positive outcome, improvement could not yet be established, a finding that seems to contradict the pronounced improved DROP-IN detection rate as reported for robotic surgery (100% versus 76%, for the DROP-IN versus rigid gamma probe respectively) [23]. Comparing the approaches shows there are two fundamental differences between non-robotic and robotic laparoscopic procedures. Firstly, in the non-robotic laparoscopic setting, probe insertion and handling are performed by the surgeon and lack of maneuverability can be overcome by inserting the probe via different trocars; however, in the current study, only the assistant trocar was large enough for probe insertion. In the robotic setting, rigid gamma probe use is often completely restricted to the assistant trocar: as the robotic platform fixes itself to the majority of the trocars (called “robot docking”), generally only one trocar is available for gamma tracing (i.e., an assistant trocar located at the side) (Fig. 5) [21], a feature that limits nodal identification on the ipsilateral side of the patient [23]. Secondly, in the robotic setting, the surgeon is no longer present at the sterile bedside, preventing autonomous tracing with the rigid probe [21], thereby making the procedure and the handling of the probe highly reliant on the communication between the surgeon and the bedside assistant. A shortcoming that is overcome with the DROP-IN probe is that this latter technology can be directly manipulated by the robotic surgeon. In addition, indicating autonomy in gamma probe use by the surgeon is a major driver for its accuracy. Prospective randomized evaluations in larger patient cohorts, and perhaps additional clinical indications, will help to further understand which features offer advantages with regard to (quantifiable) surgical outcome.
Differences in the traditional laparoscopic operating room versus robotic operating room. A In the traditional laparoscopic setting, trocar placement is less restricted than in the robotic setting. If the size of the trocars used permits it, this could allow for gamma probe insertion at various points of entrance in to the patient. B In the robotic setting, most trocars are in permanent use by the robot, limiting probe access to the assistant trocar only. C Schematic overview of DROP-IN probe maneuverability with rigid laparoscopic instruments. D Schematic overview of DROP-IN probe maneuverability with robotic instruments
As reported by Paredes et al. and Sanches et al., the hybrid radio-and fluorescence-guidance concept enables fluorescence-based SLN confirmation in the majority of cases for both cervical and endometrial cancer [32, 33]. However, in line with previous reports [23, 24], DROP-IN radioguidance outperformed fluorescence imaging (96% versus 85%, in the current study respectively), an effect that is likely due to the in-depth SLN detection capabilities of gamma probe detection, as opposed to the superficial nature of fluorescence imaging [36].
DROP-IN usage with conventional rigid laparoscopic instruments was not evaluated in this study. As illustrated in Fig. 5C, such a setup would be possible, but would not increase the DoF available for tracing as compared to the rigid laparoscopic gamma probe (i.e., 4 DoF; Fig. 1A). Given the reported findings, one could theorize also a similar detection rate would be found with a DROP-IN + traditional instrument combination. However, looking at a different aspect of the surgical procedure, namely surgical logistics, a possible advantage of using the DROP-IN probe is also provided by the fact that it can stay in situ during the procedure, meaning it can simply be picked up whenever the surgeon needs it. A recent study indicates that kinematic assessments of the intraoperative DROP-IN probe movements can be used to objectively assess how well the probe use relates to the surgical workflow [37, 38]. Perhaps, future developments of such intraoperative kinematic assessments could eventually also be used to translate surgical preference for improved logistics and maneuverability into quantifiable surgical performance improvements as provided by the novel technologies.
There is a continuing trend towards increasingly minimal-invasive methodologies [39]. However, to realize true precision surgery, precise surgical instruments should be complemented with precise target definition by molecular detection (i.e., miniaturized detection, steerability, augmented data display) [21], innovations that are supported by the advances made in radiochemistry (i.e., novel (tumor-targeted) radiopharmaceuticals, e.g., 99mTc-PSMA I&S [40], 99mTc-folate [41], 99mTc-FAPI-34 [42], 99mTc- or 111In-labeled somatostatin analogs [43]). By actively pursuing both the engineering and chemical tracks, the field of radioguided surgery is rapidly changing the way surgery can be performed.
Conclusion
We have successfully demonstrated the use of steerable DROP-IN radioguidance during laparoscopic cervical and endometrial cancer SLN procedures, thereby expanding the DROP-IN utility beyond robotic procedures. The find rate with the steerable approach was equal to that of the traditional rigid gamma probe, but came with a slightly superior surgical experience. These findings encourage further investigation and consideration on a path towards routine clinical practice and improved patient outcome.
References
Khan SR, Rockall AG, Barwick TD. Molecular imaging in cervical cancer. Q J Nucl Med Mol Imaging. 2016;60:77–92.
Cibula D, Kocian R, Plaikner A, Jarkovsky J, Klat J, Zapardiel I, et al. Sentinel lymph node mapping and intraoperative assessment in a prospective, international, multicentre, observational trial of patients with cervical cancer: the SENTIX trial. Eur J Cancer. 2020;137:69–80.
van Leeuwen FW, Winter A, van Der Poel HG, Eiber M, Suardi N, Graefen M, et al. Technologies for image-guided surgery for managing lymphatic metastases in prostate cancer. Nat Rev Urol. 2019;16:159–71.
Cibula D, Oonk MH, Abu-Rustum NR. Sentinel lymph node biopsy in the management of gynecologic cancer. Curr Opin Gynecol Obstet. 2015;27:66–72.
Hoogendam JP, Veldhuis WB, Hobbelink MG, Verheijen RH, van den Bosch MA, Zweemer RP. 99mTc SPECT/CT versus planar lymphoscintigraphy for preoperative sentinel lymph node detection in cervical cancer: a systematic review and metaanalysis. J Nucl Med. 2015;56:675–80.
Kim CH, Soslow RA, Park KJ, Barber EL, Khoury-Collado F, Barlin JN, et al. Pathologic ultrastaging improves micrometastasis detection in sentinel lymph nodes during endometrial cancer staging. Int J Gynecol Cancer. 2013;23:964–70.
Wit EM, KleinJan GH, Berrens A-C, van Vliet R, van Leeuwen PJ, Buckle T, et al. A hybrid radioactive and fluorescence approach is more than the sum of its parts; outcome of a phase II randomized sentinel node trial in prostate cancer patients. Eur J Nucl Med Mol Imaging. 2023;50:2861–71.
Giammarile F, Vidal-Sicart S, Paez D, Pellet O, Enrique E-L, Mikhail-Lette M, et al. Sentinel lymph node methods in breast cancer. Semin Nucl Med. 2022;52:551–60.
Wright F, Souter L, Kellett S, Easson A, Murray C, Toye J, et al. Primary excision margins, sentinel lymph node biopsy, and completion lymph node dissection in cutaneous melanoma: a clinical practice guideline. Curr Oncol. 2019;26:541–50.
Brouwer OR, Albersen M, Parnham A, Protzel C, Pettaway CA, Ayres B, et al. European Association of Urology-American Society of Clinical Oncology collaborative guideline on penile cancer: 2023 update. Eur Urol. 2023;83:548–60.
Małkiewicz B, Kiełb P, Kobylański M, Karwacki J, Poterek A, Krajewski W, et al. Sentinel lymph node techniques in urologic oncology: current knowledge and application. Cancers. 2023;15:2495.
Balaya V, Guani B, Morice P, Querleu D, Fourchotte V, Leblanc E, et al. Long-term oncological safety of sentinel lymph node biopsy in early-stage cervical cancer: a post-hoc analysis of SENTICOL I and SENTICOL II cohorts. Gynecol Oncol. 2022;164:53–61.
Nagar H, Wietek N, Goodall RJ, Hughes W, Schmidt-Hansen M, Morrison J. Sentinel node biopsy for diagnosis of lymph node involvement in endometrial cancer. Cochrane Database Syst Rev. 2021;6:Cd013021.
Sinha A, West A, Hayes J, Teoh J, Decaestecker K, Vasdev N. Methods of sentinel lymph node detection and management in urinary bladder cancer—a narrative review. Curr Oncol. 2022;29:1335–48.
Booka E, Takeuchi H. Recent advances in sentinel node navigation surgery for early gastric cancer. J Gastric Cancer. 2023;23:159.
Povoski SP, Neff RL, Mojzisik CM, O’Malley DM, Hinkle GH, Hall NC, et al. A comprehensive overview of radioguided surgery using gamma detection probe technology. World J Surg Oncol. 2009;7:1–63.
Van Oosterom MN, Rietbergen DD, Welling MM, Van Der Poel HG, Maurer T, Van Leeuwen FW. Recent advances in nuclear and hybrid detection modalities for image-guided surgery. Expert Rev Med Devices. 2019;16:711–34.
van der Poel HG, Buckle T, Brouwer OR, Olmos RAV, van Leeuwen FW. Intraoperative laparoscopic fluorescence guidance to the sentinel lymph node in prostate cancer patients: clinical proof of concept of an integrated functional imaging approach using a multimodal tracer. Eur Urol. 2011;60:826–33.
Alkatout I, Mechler U, Mettler L, Pape J, Maass N, Biebl M, et al. The development of laparoscopy—a historical overview. Front Surg. 2021;8:799442.
Heller S, Zanzonico P. Nuclear probes and intraoperative gamma cameras. Semin Nucl Med. 2011;41:166–81.
van Oosterom MN, Azargoshasb S, Slof LJ, van Leeuwen FW. Robotic radioguided surgery: toward full integration of radio-and hybrid-detection modalities. Clin Transl Imaging. 2023;11:533–44.
van Oosterom MN, Simon H, Mengus L, Welling MM, van der Poel HG, van den Berg NS, et al. Revolutionizing (robot-assisted) laparoscopic gamma tracing using a drop-in gamma probe technology. Am J Nucl Med Mol Imaging. 2016;6:1.
Dell’Oglio P, Meershoek P, Maurer T, Wit EM, van Leeuwen PJ, van der Poel HG, et al. A DROP-IN gamma probe for robot-assisted radioguided surgery of lymph nodes during radical prostatectomy. Eur Urol. 2021;79:124–32.
Meershoek P, van Oosterom MN, Simon H, Mengus L, Maurer T, van Leeuwen PJ, et al. Robot-assisted laparoscopic surgery using DROP-IN radioguidance: first-in-human translation. Eur J Nucl Med Mol Imaging. 2019;46:49–53.
Gandaglia G, Mazzone E, Stabile A, Pellegrino A, Cucchiara V, Barletta F, et al. Prostate-specific membrane antigen radioguided surgery to detect nodal metastases in primary prostate cancer patients undergoing robot-assisted radical prostatectomy and extended pelvic lymph node dissection: results of a planned interim analysis of a prospective phase 2 study. Eur Urol. 2022;82:411–8.
Junquera JMA, Harke NN, Walz JC, Hadaschik B, Adshead J, Everaerts W, et al. A drop-in gamma probe for minimally invasive sentinel lymph node dissection in prostate cancer: preclinical evaluation and interim results from a multicenter clinical trial. Clin Nucl Med. 2023;48:213–20.
Junquera JMA, Mestre-Fusco A, Grootendorst MR, Vidal-Sicart S, Fumado L. Sentinel lymph node biopsy in prostate cancer using the SENSEI® drop-in gamma probe. Clin Nucl Med. 2022;47:86–7.
Gondoputro W, Scheltema MJ, Blazevski A, Doan P, Thompson JE, Amin A, et al. Robot-assisted prostate-specific membrane antigen–radioguided surgery in primary diagnosed prostate cancer. J Nucl Med. 2022;63:1659–64.
de Barros HA, van Oosterom MN, Donswijk ML, Hendrikx JJ, Vis AN, Maurer T, et al. Robot-assisted prostate-specific membrane antigen–radioguided salvage surgery in recurrent prostate cancer using a DROP-IN gamma probe: the first prospective feasibility study. Eur Urol. 2022;82:97–105.
van Leeuwen FW, van Oosterom MN, Meershoek P, van Leeuwen PJ, Berliner C, van der Poel HG, et al. Minimal-invasive robot-assisted image-guided resection of prostate-specific membrane antigen–positive lymph nodes in recurrent prostate cancer. Clin Nucl Med. 2019;44:580–1.
Baeten IG, Hoogendam JP, Braat AJ, Zweemer RP, Gerestein CG. Feasibility of a drop-in γ-probe for radioguided sentinel lymph detection in early-stage cervical cancer. EJNMMI Res. 2022;12:36.
Paredes P, Vidal-Sicart S, Campos F, Tapias A, Sánchez N, Martínez S, et al. Role of ICG-99m Tc-nanocolloid for sentinel lymph node detection in cervical cancer: a pilot study. Eur J Nucl Med Mol Imaging. 2017;44:1853–61.
Sánchez-Izquierdo N, Vidal-Sicart S, Campos F, Torné A, Angeles MA, Migliorelli F, et al. Detection of the sentinel lymph node with hybrid tracer (ICG-[99mTc] Tc-albumin nanocolloid) in intermediate-and high-risk endometrial cancer: a feasibility study. EJNMMI Res. 2021;11:1–13.
Vermeeren L, van der Ploeg IM, Olmos RAV, Meinhardt W, Klop WMC, Kroon BB, et al. SPECT/CT for preoperative sentinel node localization. J Surg Oncol. 2010;101:184–90.
Balaya V, Guani B, Benoit L, Magaud L, Bonsang-Kitzis H, Ngô C, et al. Diagnostic value of frozen section examination of sentinel lymph nodes in early-stage cervical cancer at the time of ultrastaging. Gynecol Oncol. 2020;158:576–83.
van Oosterom MN, Meershoek P, Welling MM, Pinto F, Matthies P, Simon H, et al. Extending the hybrid surgical guidance concept with freehand fluorescence tomography. IEEE Trans Med Imaging. 2019;39:226–35.
Azargoshasb S, de Barros HA, Rietbergen DD, Dell’Oglio P, van Leeuwen PJ, Wagner C, et al. Artificial intelligence-supported video analysis as a means to assess the impact of DROP-IN image guidance on robotic surgeons: radioguided sentinel lymph node versus PSMA-targeted prostate cancer surgery. Adv Intell Syst. 2023;5:2300192.
Azargoshasb S, van Alphen S, Slof LJ, Rosiello G, Puliatti S, van Leeuwen SI, et al. The Click-On gamma probe, a second-generation tethered robotic gamma probe that improves dexterity and surgical decision-making. Eur J Nucl Med Mol Imaging. 2021;48:4142–51.
Meeuwsen F, Van Luyn F, Blikkendaal M, Jansen F, Van den Dobbelsteen J. Surgical phase modelling in minimal invasive surgery. Surg Endosc. 2019;33:1426–32.
Robu S, Schottelius M, Eiber M, Maurer T, Gschwend J, Schwaiger M, et al. Preclinical evaluation and first patient application of 99mTc-PSMA-I&S for SPECT imaging and radioguided surgery in prostate cancer. J Nucl Med. 2017;58:235–42.
Upadhaya P, Hazari PP, Mishra AK, Dutta B, Hassan P, Patravale V. Radiolabelled folate micellar carriers as proposed diagnostic aid for CNS tumors by nasal route. Drug Deliv Transl Res. 2023;13:2604–13.
Lindner T, Altmann A, Krämer S, Kleist C, Loktev A, Kratochwil C, et al. Design and development of 99mTc-labeled FAPI tracers for SPECT imaging and 188Re therapy. J Nucl Med. 2020;61:1507–13.
Cockburn KC, Toumi Z, Mackie A, Julyan P. Radioguided surgery for gastroenteropancreatic neuroendocrine tumours: a systematic literature review. J Gastrointest Surg. 2021;25:3244–57.
Funding
This research was supported by a Dutch Research Council NWO-TTW-VICI grant (TTW 16141), a NWO KICH grant (KICH1.ST03.21.030), and the Sociedad Española de Medicina Nuclear e Imagen Molecular SEMNIM-Janssen-Cilag S.A. (2021) grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. It was approved by the local ethical committee (HCB/2021/0777 and NCT04492995).
Consent to participate and for publication
Informed consent was obtained from all individual patients included.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
van Oosterom, M.N., Diaz-Feijóo, B., Santisteban, M.I. et al. Steerable DROP-IN radioguidance during minimal-invasive non-robotic cervical and endometrial sentinel lymph node surgery. Eur J Nucl Med Mol Imaging (2024). https://doi.org/10.1007/s00259-023-06589-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00259-023-06589-3