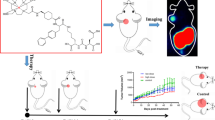
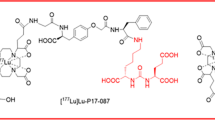
Abstract
Purpose
This study aimed to explore the feasibility of using [177Lu]Lu-prostate-specific membrane antigen (PSMA)-617 and [177Lu]Lu-Evans blue (EB)-PSMA-617 for in vivo radioligand therapy by single-dose administration in a PSMA-positive hepatocellular carcinoma (HCC) xenograft mouse model.
Methods
[177Lu]Lu-PSMA-617 and [177Lu]Lu-EB-PSMA-617 were prepared, and labelling efficiency and radiochemical purity were determined. A HepG2 human HCC subcutaneous xenograft mouse model was established. After intravenous injection of [177Lu]Lu-PSMA-617 or [177Lu]Lu-EB-PSMA-617 (37 MBq) into the mouse model, single-photon emission computed tomography/computed tomography (SPECT/CT) was performed. Biodistribution studies were conducted to verify targeting specificity and pharmacokinetics. In the radioligand therapy study, mice were randomized into 4 groups: 37 MBq [177Lu]Lu-PSMA-617, 18.5 MBq [177Lu]Lu-PSMA-617, 7.4 MBq [177Lu]Lu-EB-PSMA-617, and saline (control). A single-dose administration was applied at the beginning of therapy studies. Tumor volume, body weight, and survival were monitored every 2 days. After the end of therapy, mice were euthanized. Tumors were then weighed, and systemic toxicity was evaluated via blood testing and histological examination of healthy organs.
Results
[177Lu]Lu-PSMA-617 and [177Lu]Lu-EB-PSMA-617 were successfully prepared with high purity and stability. SPECT/CT and biodistribution showed that tumor uptake was higher and persisted longer for [177Lu]Lu-EB-PSMA-617 compared with [177Lu]Lu-PSMA-617. [177Lu]Lu-PSMA-617 was rapidly cleared from the blood, while [177Lu]Lu-EB-PSMA-617 persisted for significantly longer. In radioligand therapy studies, tumor growth was significantly suppressed in the 37 MBq [177Lu]Lu-PSMA-617, 18.5 MBq [177Lu]Lu-PSMA-617, and 7.4 MBq [177Lu]Lu-EB-PSMA-617 groups compared to the saline group. Median survival was 40, 44, 43, and 30 days, respectively. No healthy organ toxicity was observed in safety and tolerability evaluation.
Conclusions
Radioligand therapy using [177Lu]Lu-PSMA-617 and [177Lu]Lu-EB-PSMA-617 significantly suppressed tumor growth and prolonged survival time in PSMA-positive HCC xenograft mice without obvious toxicity. These radioligands appear promising for clinical use in humans, and future studies are warranted.







Similar content being viewed by others
Data Availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary information.
References
European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu; European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. https://doi.org/10.1016/j.jhep.2018.03.019.
Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301–14. https://doi.org/10.1016/S0140-6736(18)30010-2.
Zaydfudim VM, Vachharajani N, Klintmalm GB, Jarnagin WR, Hemming AW, Doyle MB, Cavaness KM, Chapman WC, Nagorney DM. Liver Resection and Transplantation for Patients With Hepatocellular Carcinoma Beyond Milan Criteria. Ann Surg. 2016;264(4):650–8. https://doi.org/10.1097/SLA.0000000000001866.
Mazzaferro V, Bhoori S, Sposito C, Bongini M, Langer M, Miceli R, Mariani L. Milan criteria in liver transplantation for hepatocellular carcinoma: an evidence-based analysis of 15 years of experience. Liver Transpl. 2011;17(Suppl 2):S44-57. https://doi.org/10.1002/lt.22365.
Molla N, AlMenieir N, Simoneau E, Aljiffry M, Valenti D, Metrakos P, Boucher LM, Hassanain M. The role of interventional radiology in the management of hepatocellular carcinoma. Curr Oncol. 2014;21(3):e480–92. https://doi.org/10.3747/co.21.1829.
Gish RG, Porta C, Lazar L, Ruff P, Feld R, Croitoru A, Feun L, Jeziorski K, Leighton J, Gallo J, Kennealey GT. Phase III randomized controlled trial comparing the survival of patients with unresectable hepatocellular carcinoma treated with nolatrexed or doxorubicin. J Clin Oncol. 2007;25(21):3069–75. https://doi.org/10.1200/JCO.2006.08.4046.
Yeo W, Mok TS, Zee B, Leung TW, Lai PB, Lau WY, Koh J, Mo FK, Yu SC, Chan AT, Hui P, Ma B, Lam KC, Ho WM, Wong HT, Tang A, Johnson PJ. A randomized phase III study of doxorubicin versus cisplatin/interferon alpha-2b/doxorubicin/fluorouracil (PIAF) combination chemotherapy for unresectable hepatocellular carcinoma. J Natl Cancer Inst. 2005;97(20):1532–8. https://doi.org/10.1093/jnci/dji315.
Edeline J, Raoul JL, Vauleon E, Guillygomac’h A, Boudjema K, Boucher E. Systemic chemotherapy for hepatocellular carcinoma in non-cirrhotic liver: a retrospective study. World J Gastroenterol. 2009;15(6):713–6. https://doi.org/10.3748/wjg.15.713.
Kraeber-Bodéré F, Bodet-Milin C, Rousseau C, Eugène T, Pallardy A, Frampas E, Carlier T, Ferrer L, Gaschet J, Davodeau F, Gestin JF, Faivre-Chauvet A, Barbet J, Chérel M. Radioimmunoconjugates for the treatment of cancer. Semin Oncol. 2014;41(5):613–22. https://doi.org/10.1053/j.seminoncol.2014.07.004.
Mier W, Kratochwil C, Hassel JC, Giesel FL, Beijer B, Babich JW, Friebe M, Eisenhut M, Enk A, Haberkorn U. Radiopharmaceutical therapy of patients with metastasized melanoma with the melanin-binding benzamide 131I-BA52. J Nucl Med. 2014;55(1):9–14. https://doi.org/10.2967/jnumed.112.112789.
van der Zwan WA, Bodei L, Mueller-Brand J, de Herder WW, Kvols LK, Kwekkeboom DJ. GEPNETs update: Radionuclide therapy in neuroendocrine tumors. Eur J Endocrinol. 2015;172(1):R1-8. https://doi.org/10.1530/EJE-14-0488.
Bodei L, Kidd M, Paganelli G, Grana CM, Drozdov I, Cremonesi M, Lepensky C, Kwekkeboom DJ, Baum RP, Krenning EP, Modlin IM. Long-term tolerability of PRRT in 807 patients with neuroendocrine tumours: the value and limitations of clinical factors. Eur J Nucl Med Mol Imaging. 2015;42(1):5–19. https://doi.org/10.1007/s00259-014-2893-5.
Elsasser-Beile U, Buhler P, Wolf P. Targeted therapies for prostate cancer against the prostate specific membrane antigen. Curr Drug Targets. 2009;10:118–25. https://doi.org/10.2174/138945009787354601.
Bühler P, Wolf P, Elsässer-Beile U. Targeting the prostate-specific membrane antigen for prostate cancer therapy. Immunotherapy. 2009;1:471–81. https://doi.org/10.2217/imt.09.17.
Slovin SF. Targeting novel antigens for prostate cancer treatment: Focus on prostate-specific membrane antigen. Expert Opin Ther Targets. 2005;9:561–70. https://doi.org/10.1517/14728222.9.3.561.
Chang SS, Reuter VE, Heston WD, Bander NH, Grauer LS, Gaudin PB. Five different anti-prostate- specific membrane antigen (PSMA) antibodies confirm PSMA expression in tumor-associated neovasculature. Cancer Res. 1999;59:3192–8.
Wernicke AG, Varma S, Greenwood EA, Christos PJ, Chao KS, Liu H, et al. Prostate-specific membrane antigen expression in tumor-associated vasculature of breast cancers. APMIS. 2014;122(6):482–9. https://doi.org/10.1111/apm.12195.
Baccala A, Sercia L, Li J, Heston W, Zhou M. Expression of prostate-specific membrane antigen in tumor-associated neovasculature of renal neoplasms. Urology. 2007;70(2):385–90. https://doi.org/10.1016/j.urology.2007.03.025.
Haffner MC, Kronberger IE, Ross JS, Sheehan CE, Zitt M, Mühlmann G, et al. Prostate-specific membrane antigen expression in the neovasculature of gastric and colorectal cancers. Hum Pathol. 2009;40(12):1754–61. https://doi.org/10.1016/j.humpath.2009.06.003.
Wang HL, Wang SS, Song WH, Pan Y, Yu HP, Si TG, et al. Expression of prostate-specific membrane antigen in lung cancer cells and tumor neovasculature endothelial cells and its clinical significance. PLoS One. 2015;10(5):e0125924. https://doi.org/10.1371/journal.pone.0125924.
Kasoha M, Unger C, Solomayer EF, Bohle RM, Zaharia C, Khreich F, et al. Prostate-specific membrane antigen (PSMA) expression in breast cancer and its metastases. Clin Exp Metastasis. 2017;34(8):479–90. https://doi.org/10.1007/s10585-018-9878-x.
Denmeade SR, Mhaka AM, Rosen DM, Brennen WN, Dalrymple S, Dach I, et al. Engineering a prostate-specific membrane antigen-activated tumor endothelial cell prodrug for cancer therapy. Sci Transl Med. 2012;4(140):140ra86.
Patel D, Loh H, Le K, Stevanovic A, Mansberg R. Incidental Detection of Hepatocellular Carcinoma on 68Ga-Labeled Prostate-Specific Membrane Antigen PET/CT. Clin Nucl Med. 2017;42(11):881–4. https://doi.org/10.1097/RLU.0000000000001832.
Taneja S, Taneja R, Kashyap V, Jha A, Jena A. 68Ga-PSMA Uptake in Hepatocellular Carcinoma. Clin Nucl Med. 2017;42(1):e69–70. https://doi.org/10.1097/RLU.0000000000001355.
Sasikumar A, Joy A, Nanabala R, Pillai MR, Thomas B, Vikraman KR. (68)Ga-PSMA PET/CT imaging in primary hepatocellular carcinoma. Eur J Nucl Med Mol Imaging. 2016;43(4):795–6. https://doi.org/10.1007/s00259-015-3297-x.
Kuyumcu S, Has-Simsek D, Iliaz R, Sanli Y, Buyukkaya F, Akyuz F, et al. Evidence of Prostate-Specific Membrane Antigen Expression in Hepatocellular Carcinoma Using 68Ga-PSMA PET/CT. Clin Nucl Med. 2019;44(9):702–6. https://doi.org/10.1097/RLU.0000000000002701.
Kesler M, Levine C, Hershkovitz D, Mishani E, Menachem Y, Lerman H, et al. 68Ga-PSMA is a novel PET-CT tracer for imaging of hepatocellular carcinoma: A prospective pilot study. J Nucl Med. 2019;60(2):185–91. https://doi.org/10.2967/jnumed.118.214833.
Rahbar K, Ahmadzadehfar H, Kratochwil C, Haberkorn U, Schäfers M, Essler M, et al. German Multicenter Study Investigating 177Lu-PSMA-617 Radioligand Therapy in Advanced Prostate Cancer Patients. J Nucl Med. 2017;58(1):85–90. https://doi.org/10.2967/jnumed.116.183194.
Rahbar K, Schmidt M, Heinzel A, Eppard E, Bode A, Yordanova A, et al. Response and Tolerability of a Single Dose of 177Lu-PSMA-617 in Patients with Metastatic Castration-Resistant Prostate Cancer: A Multicenter Retrospective Analysis. J Nucl Med. 2016;57(9):1334–8. https://doi.org/10.2967/jnumed.116.173757.
Rahbar K, Boegemann M, Yordanova A, Eveslage M, Schäfers M, Essler M, et al. PSMA targeted radioligandtherapy in metastatic castration resistant prostate cancer after chemotherapy, abiraterone and/or enzalutamide. A retrospective analysis of overall survival. Eur J Nucl Med Mol Imaging. 2018;45(1):12–9. https://doi.org/10.1007/s00259-017-3848-4.
Ahmadzadehfar H, Eppard E, Kürpig S, Fimmers R, Yordanova A, Schlenkhoff CD, et al. Therapeutic response and side effects of repeated radioligand therapy with 177Lu-PSMA-DKFZ-617 of castrate-resistant metastatic prostate cancer. Oncotarget. 2016;7(11):12477–88. https://doi.org/10.18632/oncotarget.7245.
Yordanova A, Linden P, Hauser S, Meisenheimer M, Kürpig S, Feldmann G, et al. Outcome and safety of rechallenge [177Lu]Lu-PSMA-617 in patients with metastatic prostate cancer. Eur J Nucl Med Mol Imaging. 2019;46(5):1073–80. https://doi.org/10.1007/s00259-018-4222-x.
Khreish F, Kochems N, Rosar F, Sabet A, Ries M, Maus S, et al. Response and outcome of liver metastases in patients with metastatic castration-resistant prostate cancer (mCRPC) undergoing 177Lu-PSMA-617 radioligand therapy. Eur J Nucl Med Mol Imaging. 2021;48(1):103–12. https://doi.org/10.1007/s00259-020-04828-5.
Kratochwil C, Giesel FL, Eder M, Afshar-Oromieh A, Benešová M, Mier W, et al. [177Lu]Lutetium-labelled PSMA ligand-induced remission in a patient with metastatic prostate cancer. Eur J Nucl Med Mol Imaging. 2015;42(6):987–8. https://doi.org/10.1007/s00259-014-2978-1.
Hillier SM, Maresca KP, Femia FJ, Marquis JC, Foss CA, Nguyen N, et al. Preclinical evaluation of novel glutamate-urea-lysine analogues that target prostate-specific membrane antigen as molecular imaging pharmaceuticals for prostate cancer. Cancer Res. 2009;69(17):6932–40. https://doi.org/10.1158/0008-5472.CAN-09-1682.
Violet J, Sandhu S, Iravani A, Ferdinandus J, Thang SP, Kong G, et al. Long-Term Follow-up and Outcomes of Retreatment in an Expanded 50-Patient Single-Center Phase II Prospective Trial of 177Lu-PSMA-617 Theranostics in Metastatic Castration-Resistant Prostate Cancer. J Nucl Med. 2020;61(6):857–65. https://doi.org/10.2967/jnumed.119.236414.
Gafita A, Calais J, Grogan TR, Hadaschik B, Wang H, Weber M, et al. Nomograms to predict outcomes after 177Lu-PSMA therapy in men with metastatic castration-resistant prostate cancer: an international, multicentre, retrospective study. Lancet Oncol. 2021;22(8):1115–25. https://doi.org/10.1016/S1470-2045(21)00274-6.
Sartor O, Herrmann K. Prostate Cancer Treatment: 177Lu-PSMA-617 Considerations, Concepts, and Limitations. J Nucl Med. 2022;63(6):823–9. https://doi.org/10.2967/jnumed.121.262413.
Sartor O, de Bono J, Chi KN, Fizazi K, Herrmann K, Rahbar K, et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2021;385(12):1091–103. https://doi.org/10.1056/NEJMoa2107322.
Kelly JM, Amor-Coarasa A, Nikolopoulou A, Wüstemann T, Barelli P, Kim D, et al. Dual-Target Binding Ligands with Modulated Pharmacokinetics for Endoradiotherapy of Prostate Cancer. J Nucl Med. 2017;58(9):1442–9. https://doi.org/10.2967/jnumed.116.188722.
Umbricht CA, Benešová M, Schibli R, Müller C. Preclinical Development of Novel PSMA-Targeting Radioligands: Modulation of Albumin-Binding Properties To Improve Prostate Cancer Therapy. Mol Pharm. 2018;15(6):2297–306. https://doi.org/10.1021/acs.molpharmaceut.8b00152.
Choy CJ, Ling X, Geruntho JJ, Beyer SK, Latoche JD, Langton-Webster B, et al. 177Lu-Labeled Phosphoramidate-Based PSMA Inhibitors: The Effect of an Albumin Binder on Biodistribution and Therapeutic Efficacy in Prostate Tumor-Bearing Mice. Theranostics. 2017;7(7):1928–39. https://doi.org/10.7150/thno.18719.
Kelly J, Amor-Coarasa A, Ponnala S, Nikolopoulou A, Williams C Jr, Schlyer D, et al. Trifunctional PSMA-targeting constructs for prostate cancer with unprecedented localization to LNCaP tumors. Eur J Nucl Med Mol Imaging. 2018;45(11):1841–51. https://doi.org/10.1007/s00259-018-4004-5.
Chen H, Jacobson O, Niu G, Weiss ID, Kiesewetter DO, Liu Y, et al. Novel “Add-On” Molecule Based on Evans Blue Confers Superior Pharmacokinetics and Transforms Drugs to Theranostic Agents. J Nucl Med. 2017;58(4):590–7. https://doi.org/10.2967/jnumed.116.182097.
Kulkarni HR, Singh A, Schuchardt C, Niepsch K, Sayeg M, Leshch Y, et al. PSMA-Based Radioligand Therapy for Metastatic Castration-Resistant Prostate Cancer: The Bad Berka Experience Since 2013. J Nucl Med. 2016;57(Suppl 3):97S-104S. https://doi.org/10.2967/jnumed.115.170167.
Wang Z, Tian R, Niu G, Ma Y, Lang L, Szajek LP, et al. Single Low-Dose Injection of Evans Blue Modified PSMA-617 Radioligand Therapy Eliminates Prostate-Specific Membrane Antigen Positive Tumors. Bioconjug Chem. 2018;29(9):3213–21. https://doi.org/10.1021/acs.bioconjchem.8b00556.
Wang G, Zang J, Jiang Y, Liu Q, Sui H, Wang R, et al. A single-arm, low-dose, prospective study of 177Lu-EB-PSMA radioligand therapy in patients with metastatic castration-resistant prostate cancer. J Nucl Med. 2022 Nov 3:jnumed.122.264857 https://doi.org/10.2967/jnumed.122.264857.
Zang J, Liu Q, Sui H, Wang R, Jacobson O, Fan X, et al. 177Lu-EB-PSMA Radioligand Therapy with Escalating Doses in Patients with Metastatic Castration-Resistant Prostate Cancer. J Nucl Med. 2020;61(12):1772–8. https://doi.org/10.2967/jnumed.120.242263.
Wächter S, Di Fazio P, Maurer E, Manoharan J, Keber C, Pfestroff A, et al. Prostate-Specific Membrane Antigen in Anaplastic and Poorly Differentiated Thyroid Cancer-A New Diagnostic and Therapeutic Target? Cancers (Basel). 2021;13(22):5688. https://doi.org/10.3390/cancers13225688.
de Vries LH, Lodewijk L, Braat AJAT, Krijger GC, Valk GD, Lam MGEH, et al. 68Ga-PSMA PET/CT in radioactive iodine-refractory differentiated thyroid cancer and first treatment results with 177Lu-PSMA-617. EJNMMI Res. 2020;10(1):18. https://doi.org/10.1186/s13550-020-0610-x.
Kumar A, Ballal S, Yadav MP, ArunRaj ST, Haresh KP, Gupta S, et al. 177Lu-/68Ga-PSMA Theranostics in Recurrent Glioblastoma Multiforme: Proof of Concept. Clin Nucl Med. 2020;45(12):e512–3. https://doi.org/10.1097/RLU.0000000000003142.
Kunikowska J, Charzyńska I, Kuliński R, Pawlak D, Maurin M, Królicki L. Tumor uptake in glioblastoma multiforme after IV injection of [177Lu]Lu-PSMA-617. Eur J Nucl Med Mol Imaging. 2020;47(6):1605–6. https://doi.org/10.1007/s00259-020-04715-z.
Lu Q, Long Y, Fan K, Shen Z, Gai Y, Liu Q, et al. PET imaging of hepatocellular carcinoma by targeting tumor-associated endothelium using [68Ga]Ga-PSMA-617. Eur J Nucl Med Mol Imaging. 2022;49:4000–13. https://doi.org/10.1007/s00259-022-05884-9.
Schmittgen TD, Zakrajsek BA, Hill RE, Liu Q, Reeves JJ, Axford PD, et al. Expression pattern of mouse homolog of prostate-specific membrane antigen (FOLH1) in the transgenic adenocarcinoma of the mouse prostate model. Prostate. 2003;55(4):308–16. https://doi.org/10.1002/pros.10241.
Breeman WA, van der Wansem K, Bernard BF, van Gameren A, Erion JL, Visser TJ, et al. The addition of DTPA to [177Lu-DOTA0, Tyr3] octreotate prior to administration reduces rat skeleton uptake of radioactivity. Eur J Nucl Med Mol Imaging. 2003;30(2):312–5. https://doi.org/10.1007/s00259-002-1054-4.
Acknowledgements
We would like to acknowledge the service provided by Beijing Novel Medical Equipment Ltd. for image acquisition. We thank Liwen Bianji (Edanz) (https://www.liwenbianji.cn) for editing the language of a draft of this manuscript.
Funding
This work was funded by the National Natural Science Foundation of China (No. 82030052).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All experimental schemes were performed under the guidance and approved by the Institutional Animal Care and Use Committee of Tongji Medical College of Huazhong University of Science and Technology ([2021] IACUC Number: 2688). Extensive efforts were made to ensure minimal suffering of the animals used during the study.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Theragnostic.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lu, Q., Long, Y., Gai, Y. et al. [177Lu]Lu-PSMA-617 theranostic probe for hepatocellular carcinoma imaging and therapy. Eur J Nucl Med Mol Imaging 50, 2342–2352 (2023). https://doi.org/10.1007/s00259-023-06155-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-023-06155-x