Abstract
Purpose
Current clinical and imaging tools remain suboptimal for predicting treatment response and prognosis in CNS lymphomas. We investigated the prognostic value of baseline [18F]FDG PET in patients with CNS lymphoma receiving ibrutinib-based treatments.
Methods
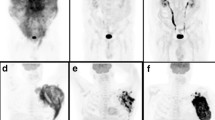
Fifty-three patients enrolled in a prospective clinical trial and underwent brain PET before receiving single-agent ibrutinib or ibrutinib in combination with methotrexate with or without rituximab. [18F]FDG uptake in these lesions was quantified by drawing PET volumes of interest around up to five [18F]FDG-avid lesions per patient (with uptake greater than surrounding brain). We measured standardized uptake values (SUVmax), metabolic tumor volumes, total lesion glycolysis (TLG), and the sum thereof in these lesions. We analyzed the relationship between PET parameters and mutation status, overall response rates, and progression-free survival (PFS).
Results
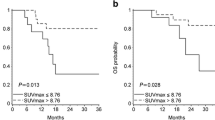
Thirty-eight patients underwent single-agent therapy and 15 received combination therapy. On PET, 15/53 patients had no measurable disease. In the other 38 patients, a total of 71 lesions were identified on PET. High-intensity [18F]FDG uptake and a larger volume of [18F]FDG-avid disease were inversely related to treatment outcome (p ≤ 0.005). In univariable analysis, PFS was linearly correlated with all PET parameters, with stronger association when sum-values were used. A multivariable model showed that risk of progression increased by 9% for every 5-unit increase in sumSUVmax (hazard ratio = 1.09 [95% CI: 1.04 to 1.14]).
Conclusion
Higher lesional metabolic parameters are inversely related to outcome in patients undergoing ibrutinib-based therapies, and sumSUVmax emerged as a strong independent prognostic factor.
Trial registration
NCT02315326; https://clinicaltrials.gov/ct2/show/NCT02315326?term=NCT02315326&draw=2&rank=1



Similar content being viewed by others
Availability of data and materials
Not applicable.
Code availability
Not applicable.
References
Ostrom QT, Gittleman H, Xu J, et al. CBTRUS Statistical Report: primary brain and other central nervous system tumors diagnosed in the United States in 2009–2013. Neuro-Oncol. 2016;18(suppl_5):v1–v75.
Mendez JS, Ostrom QT, Gittleman H, et al. The elderly left behind-changes in survival trends of primary central nervous system lymphoma over the past 4 decades. Neuro-Oncol. 2018;20(5):687–94.
Camilleri-Broët S, Martin A, Moreau A, et al. Primary central nervous system lymphomas in 72 immunocompetent patients: pathologic findings and clinical correlations. Groupe Ouest Est d’étude des Leucénies et Autres Maladies du Sang (GOELAMS). Am J Clin Pathol. 1998;110(5):607–12.
Younes A, Hilden P, Coiffier B, et al. International Working Group consensus response evaluation criteria in lymphoma (RECIL 2017). Ann Oncol. 2017;28(7):1436–47.
Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059–68.
Albano D, Bosio G, Bertoli M, Giubbini R, Bertagna F. 18F-FDG PET/CT in primary brain lymphoma. J Neuro-Oncol. 2018;136(3):577–83.
Albano D, Bertoli M, Battistotti M, et al. Prognostic role of pretreatment 18F-FDG PET/CT in primary brain lymphoma. Ann Nucl Med. 2018;32(8):532–41.
Grommes C, DeAngelis LM. Primary CNS lymphoma. J Clin Oncol. 2017;35(21):2410–8.
Grommes C, Pastore A, Palaskas N, et al. Ibrutinib unmasks critical role of Bruton tyrosine kinase in primary CNS lymphoma. Cancer Discov. 2017;7(9):1018–29.
Grommes C, Tang SS, Wolfe J, et al. Phase 1b trial of an ibrutinib-based combination therapy in recurrent/refractory CNS lymphoma. Blood. 2019;133(5):436–45.
Lionakis MS, Dunleavy K, Roschewski M, et al. Inhibition of B cell receptor signaling by ibrutinib in primary CNS lymphoma. Cancer Cell. 2017;31(6):833–843.e835.
Soussain C, Choquet S, Blonski M, et al. Ibrutinib monotherapy for relapse or refractory primary CNS lymphoma and primary vitreoretinal lymphoma: final analysis of the phase II ‘proof-of-concept’ iLOC study by the Lymphoma study association (LYSA) and the French oculo-cerebral lymphoma (LOC) network. Eur J Cancer. 2019;117:121–30.
Abrey LE, Batchelor TT, Ferreri AJ, et al. Report of an international workshop to standardize baseline evaluation and response criteria for primary CNS lymphoma. J Clin Oncol. 2005;23(22):5034–43.
Erdi YE, Mawlawi O, Larson SM, et al. Segmentation of lung lesion volume by adaptive positron emission tomography image thresholding. Cancer. 1997;80(12 Suppl):2505–9.
Hatzoglou V, Oh JH, Buck O, et al. Pretreatment dynamic contrast-enhanced MRI biomarkers correlate with progression-free survival in primary central nervous system lymphoma. J Neuro-Oncol. 2018;140(2):351–8.
Bonett DG, Wright TA. Sample size requirements for estimating Pearson, Kendall and Spearman correlations. Psychometrika. 2000;65(1):23–8.
Abrey LE, Ben-Porat L, Panageas KS, et al. Primary central nervous system lymphoma: the Memorial Sloan-Kettering Cancer Center prognostic model. J Clin Oncol. 2006;24(36):5711–5.
Abramson JS, McGree B, Noyes S, et al. Anti-CD19 CAR T cells in CNS diffuse large-B-cell lymphoma. N Engl J Med. 2017;377(8):783–4.
Frigault MJ, Dietrich J, Martinez-Lage M, et al. Tisagenlecleucel CAR T-cell therapy in secondary CNS lymphoma. Blood. 2019;134(11):860–6.
Zou Y, Tong J, Leng H, Jiang J, Pan M, Chen Z. Diagnostic value of using 18F-FDG PET and PET/CT in immunocompetent patients with primary central nervous system lymphoma: a systematic review and meta-analysis. Oncotarget. 2017;8(25):41518–28.
Mercadal S, Cortés-Romera M, Vélez P, Climent F, Gámez C, González-Barca E. Positron emission tomography combined with computed tomography in the initial evaluation and response assessment in primary central nervous system lymphoma. Med Clin. 2015;144(11):503–6.
Herhaus P, Lipkova J, Lammer F, et al. CXCR4-targeted positron emission tomography imaging of central nervous system B-cell lymphoma. J Nucl Med. 2020;61(12):1765–71.
Clarke BN. PET radiopharmaceuticals: what’s new, what’s reimbursed, and what’s next? J Nucl Med Technol. 2018. https://doi.org/10.2967/jnmt.117.205021.
Vercellino L, Cottereau AS, Casasnovas O, et al. High total metabolic tumor volume at baseline predicts survival independent of response to therapy. Blood. 2020;135(16):1396–405.
Ceriani L, Gritti G, Cascione L, et al. SAKK38/07 study: integration of baseline metabolic heterogeneity and metabolic tumor volume in DLBCL prognostic model. Blood Adv. 2020;4(6):1082–92.
Bailly C, Carlier T, Berriolo-Riedinger A, et al. Prognostic value of FDG-PET in patients with mantle cell lymphoma: results from the LyMa-PET Project. Haematologica. 2020;105(1):e33–6.
Moskowitz AJ, Schöder H, Gavane S, et al. Prognostic significance of baseline metabolic tumor volume in relapsed and refractory Hodgkin lymphoma. Blood. 2017;130(20):2196–203.
Kawai N, Zhen HN, Miyake K, Yamamaoto Y, Nishiyama Y, Tamiya T. Prognostic value of pretreatment 18F-FDG PET in patients with primary central nervous system lymphoma: SUV-based assessment. J Neuro-Oncol. 2010;100(2):225–32.
Kasenda B, Haug V, Schorb E, et al. 18F-FDG PET is an independent outcome predictor in primary central nervous system lymphoma. J Nucl Med. 2013;54(2):184–91.
Okuyucu K, Alagoz E, Ince S, Ozaydin S, Arslan N. Can metabolic tumor parameters on primary staging (18)F-FDG PET/CT aid in risk stratification of primary central nervous system lymphomas for patient management as a prognostic model? Rev Esp Med Nucl Imagen Mol. 2018;37(1):9–14.
Wilson WH, Young RM, Schmitz R, et al. Targeting B cell receptor signaling with ibrutinib in diffuse large B cell lymphoma. Nat Med. 2015;21(8):922–6.
Yamamoto T, Seino Y, Fukumoto H, et al. Over-expression of facilitative glucose transporter genes in human cancer. Biochem Biophys Res Commun. 1990;170(1):223–30.
Mathupala SP, Ko YH, Pedersen PL. Hexokinase II: cancer’s double-edged sword acting as both facilitator and gatekeeper of malignancy when bound to mitochondria. Oncogene. 2006;25(34):4777–86.
Tateishi K, Miyake Y, Kawazu M, et al. A hyperactive RelA/p65-hexokinase 2 signaling axis drives primary central nervous system lymphoma. Cancer Res. 2020;80(23):5330–43.
Acknowledgements
We thank Leah Bassity for her expert editorial support.
Funding
This work was supported by a research grant from Pharmacyclics to MSK. Pharmacyclics was not involved in the design or conduct of the study. The statistical analysis plan and data analyses were performed by MSK investigators. This study was partially supported by the National Institutes of Health/National Cancer Institute Cancer Center Support Grant (P30 CA008748), National Institutes of Health/National Cancer Institute Paul Calabresi Career Development Award for Clinical Oncology (K12 CA184746 to S.K.), and National Institutes of Health MSK SPORE in Lymphoma (P50 CA192937 to S.K.), as well as by grants from Cycle for Survival Equinox (C.G.) and the Leukemia & Lymphoma Society (C.G.).
Author information
Authors and Affiliations
Contributions
Conceived and designed the study: S.K., C.G., and H.S.; collected, analyzed, and interpreted the data: S.K., A.M., O.Y., V.H., J.H.F., L.R.S., I.K.M., C.G., and H.S.; performed statistical analysis: A.M.; wrote the manuscript: S.K., C.G., and H.S.; reviewed the data and edited and approved the final version of the manuscript: all authors.
Corresponding author
Ethics declarations
Ethical approval
All procedures involving human participants were performed in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed written consent was obtained from all individual participants included in the study.
Competing interests
C.G. reports consulting for BTG International and Kite. The remaining authors have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Oncology – Brain
Supplementary information
ESM 1
(PDF 644 kb)
Rights and permissions
About this article
Cite this article
Krebs, S., Mauguen, A., Yildirim, O. et al. Prognostic value of [18F]FDG PET/CT in patients with CNS lymphoma receiving ibrutinib-based therapies. Eur J Nucl Med Mol Imaging 48, 3940–3950 (2021). https://doi.org/10.1007/s00259-021-05386-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-021-05386-0