Abstract
Purpose
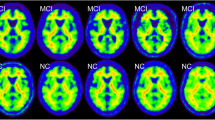
The analysis of the [11C]PiB-PET amyloid images of a unique Asian cohort of 186 participants featuring overlapping vascular diseases raised the question about the validity of current standards for amyloid quantification under abnormal conditions. In this work, we implemented a novel pipeline for improved amyloid PET quantification of this atypical cohort.
Methods
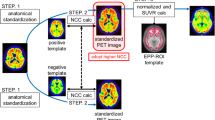
The investigated data correction and amyloid quantification methods included motion correction, standardized uptake value ratio (SUVr) quantification using the parcellated MRI (standard method) and SUVr quantification without MRI. We introduced a novel amyloid analysis method yielding 2 biomarkers: AβL which quantifies the global Aβ burden and ns that characterizes the non-specific uptake. Cut-off points were first determined using visual assessment as ground truth and then using unsupervised classification techniques.
Results
Subject’s motion impacts the accuracy of the measurement outcome but has however a limited effect on the visual rating and cut-off point determination. SUVr computation can be reliably performed for all the subjects without MRI parcellation while, when required, the parcellation failed or was of mediocre quality in 10% of the cases. The novel biomarker AβL showed an association increase of 29.5% with the cognitive tests and increased effect size between positive and negative scans compared with SUVr. ns was found sensitive to cerebral microbleeds, white matter hyperintensity, volume, and age. The cut-off points for SUVr using parcellated MRI, SUVr without parcellation, and AβL were 1.56, 1.39, and 25.5. Finally, k-means produced valid cut-off points without the requirement of visual assessment.
Conclusion
The optimal processing for the amyloid quantification of this atypical cohort allows the quantification of all the subjects, producing SUVr values and two novel biomarkers: AβL, showing important increased in their association with various cognitive tests, and ns, a parameter sensitive to non-specific retention variations caused by age and cerebrovascular diseases.





Similar content being viewed by others
Data availability
Data generated for this work and which supports the findings are available upon request.
References
Villemagne VL, Burnham S, Bourgeat P, Brown B, Ellis KA, Salvado O, et al. Amyloid beta deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer's disease: a prospective cohort study. Lancet Neurol. 2013;12:357–67. https://doi.org/10.1016/S1474-4422(13)70044-9.
Jagust W. Is amyloid-beta harmful to the brain? Insights from human imaging studies. Brain. 2016;139:23–30. https://doi.org/10.1093/brain/awv326.
Cselenyi Z, Farde L. Quantification of blood flow-dependent component in estimates of beta-amyloid load obtained using quasi-steady-state standardized uptake value ratio. J Cereb Blood Flow Metab. 2015;35:1485–93. https://doi.org/10.1038/jcbfm.2015.66.
Whittington A, Gunn RN. Alzheimer's disease neuroimaging I. amyloid load: a more sensitive biomarker for amyloid imaging. J Nucl Med. 2019;60:536–40. https://doi.org/10.2967/jnumed.118.210518.
Jack CR Jr, Wiste HJ, Weigand SD, Therneau TM, Lowe VJ, Knopman DS, et al. Defining imaging biomarker cut points for brain aging and Alzheimer’s disease. Alzheimers Dement. 2017;13:205–16. https://doi.org/10.1016/j.jalz.2016.08.005.
Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270–9. https://doi.org/10.1016/j.jalz.2011.03.008.
McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–9. https://doi.org/10.1016/j.jalz.2011.03.005.
Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K, et al. Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol. 2014;13:614–29. https://doi.org/10.1016/S1474-4422(14)70090-0.
Jack CR Jr, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14:535–62. https://doi.org/10.1016/j.jalz.2018.02.018.
Shi Y, Wardlaw JM. Update on cerebral small vessel disease: a dynamic whole-brain disease. Stroke Vasc Neurol. 2016;1:83–92. https://doi.org/10.1136/svn-2016-000035.
Villeneuve S, Rabinovici GD, Cohn-Sheehy BI, Madison C, Ayakta N, Ghosh PM, et al. Existing Pittsburgh compound-B positron emission tomography thresholds are too high: statistical and pathological evaluation. Brain. 2015;138:2020–33. https://doi.org/10.1093/brain/awv112.
Feng L, Chong MS, Lim WS, Ng TP. The Modified Mini-Mental State Examination test: normative data for Singapore Chinese older adults and its performance in detecting early cognitive impairment. Singap Med J. 2012;53:458–62.
Hilal S, Sikking E, Shaik MA, Chan QL, van Veluw SJ, Vrooman H, et al. Cortical cerebral microinfarcts on 3T MRI: a novel marker of cerebrovascular disease. Neurology. 2016;87:1583–90. https://doi.org/10.1212/WNL.0000000000003110.
Delso G, Furst S, Jakoby B, Ladebeck R, Ganter C, Nekolla SG, et al. Performance measurements of the Siemens mMR integrated whole-body PET/MR scanner. J Nucl Med. 2011;52:1914–22. https://doi.org/10.2967/jnumed.111.092726.
Reilhac A, Merida I, Irace Z, Stephenson MC, Weekes AA, Chen C, et al. Development of a dedicated rebinner with rigid motion correction for the mMR PET/MR scanner, and validation in a large cohort of (11)C-PIB scans. J Nucl Med. 2018;59:1761–7. https://doi.org/10.2967/jnumed.117.206375.
Panin VY, Kehren F, Michel C, Casey M. Fully 3-D PET reconstruction with system matrix derived from point source measurements. IEEE Trans Med Imaging. 2006;25:907–21.
Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12:822–38. https://doi.org/10.1016/S1474-4422(13)70124-8.
Vrooman HA, Cocosco CA, van der Lijn F, Stokking R, Ikram MA, Vernooij MW, et al. Multi-spectral brain tissue segmentation using automatically trained k-nearest-neighbor classification. Neuroimage. 2007;37:71–81. https://doi.org/10.1016/j.neuroimage.2007.05.018.
Hilal S, Saini M, Tan CS, Catindig JA, Dong YH, Holandez RL, et al. Intracranial stenosis, cerebrovascular diseases, and cognitive impairment in Chinese. Alzheimer Dis Assoc Disord. 2015;29:12–7. https://doi.org/10.1097/WAD.0000000000000045.
Ng S, Villemagne VL, Berlangieri S, Lee ST, Cherk M, Gong SJ, et al. Visual assessment versus quantitative assessment of 11C-PIB PET and 18F-FDG PET for detection of Alzheimer’s disease. J Nucl Med. 2007;48:547–52.
Yamane T, Ishii K, Sakata M, Ikari Y, Nishio T, Ishii K, et al. Inter-rater variability of visual interpretation and comparison with quantitative evaluation of (11)C-PiB PET amyloid images of the Japanese Alzheimer’s Disease Neuroimaging Initiative (J-ADNI) multicenter study. Eur J Nucl Med Mol Imaging. 2017;44:850–7. https://doi.org/10.1007/s00259-016-3591-2.
Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–74.
Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage. 2011;54:2033–44. https://doi.org/10.1016/j.neuroimage.2010.09.025.
Joachim CL, Morris JH, Selkoe DJ. Diffuse senile plaques occur commonly in the cerebellum in Alzheimer’s disease. Am J Pathol. 1989;135:309–19.
Lee SH, Bae HJ, Yoon BW, Kim H, Kim DE, Roh JK. Low concentration of serum total cholesterol is associated with multifocal signal loss lesions on gradient-echo magnetic resonance imaging: analysis of risk factors for multifocal signal loss lesions. Stroke. 2002;33:2845–9. https://doi.org/10.1161/01.str.0000036092.23649.2e.
Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh compound-B. Ann Neurol. 2004;55:306–19. https://doi.org/10.1002/ana.20009.
Fodero-Tavoletti MT, Rowe CC, McLean CA, Leone L, Li QX, Masters CL, et al. Characterization of PiB binding to white matter in Alzheimer disease and other dementias. J Nucl Med. 2009;50:198–204. https://doi.org/10.2967/jnumed.108.057984.
Iwamoto N, Nishiyama E, Ohwada J, Arai H. Distribution of amyloid deposits in the cerebral white matter of the Alzheimer’s disease brain: relationship to blood vessels. Acta Neuropathol. 1997;93:334–40. https://doi.org/10.1007/s004010050624.
Behrouz N, Defossez A, Delacourte A, Mazzuca M. Cortical beta-amyloid. Nature. 1990;344:497. https://doi.org/10.1038/344497a0.
Lockhart A, Lamb JR, Osredkar T, Sue LI, Joyce JN, Ye L, et al. PIB is a non-specific imaging marker of amyloid-beta (Abeta) peptide-related cerebral amyloidosis. Brain. 2007;130:2607–15. https://doi.org/10.1093/brain/awm191.
Lowe VJ, Lundt ES, Senjem ML, Schwarz CG, Min HK, Przybelski SA, et al. White matter reference region in PET studies of (11)C-Pittsburgh compound B uptake: effects of age and amyloid-beta deposition. J Nucl Med. 2018;59:1583–9. https://doi.org/10.2967/jnumed.117.204271.
Glodzik L, Rusinek H, Li J, Zhou C, Tsui W, Mosconi L, et al. Reduced retention of Pittsburgh compound B in white matter lesions. Eur J Nucl Med Mol Imaging. 2015;42:97–102. https://doi.org/10.1007/s00259-014-2897-1.
Goodheart AE, Tamburo E, Minhas D, Aizenstein HJ, McDade E, Snitz BE, et al. Reduced binding of Pittsburgh compound-B in areas of white matter hyperintensities. Neuroimage Clin. 2015;9:479–83. https://doi.org/10.1016/j.nicl.2015.09.009.
Hashimoto T, Yokota C, Koshino K, Temma T, Yamazaki M, Iguchi S, et al. Binding of (11)C-Pittsburgh compound-B correlated with white matter injury in hypertensive small vessel disease. Ann Nucl Med. 2017;31:227–34. https://doi.org/10.1007/s12149-017-1152-9.
Yates PA, Desmond PM, Phal PM, Steward C, Szoeke C, Salvado O, et al. Incidence of cerebral microbleeds in preclinical Alzheimer disease. Neurology. 2014;82:1266–73. https://doi.org/10.1212/WNL.0000000000000285.
Acknowledgments
We acknowledge all the NUH memory clinic and Memory Aging and Cognition Centre coordinators for their contributions to subject recruitment and data acquisition.
Funding
This study was supported by the following National Medical Research Council grants: NMRC/CG/NUHS/2010 - R-184-005-184-511, NMRC/CG/013/2013, and NMRC/CIRG/1446/2016.
Author information
Authors and Affiliations
Contributions
TT, YHN, FNS, SV, BG, CPC, and AR developed the study concept. MRI data were collected and analyzed by SH, BG, and HV. PET data were analyzed by MCS, YHN, DK, AAW, and AR. Data simulation, analysis, and reporting were done by TT. The first draft of the manuscript was written by TT and AR, with critical revisions by YHN, FNS, SH, BG, MI, JJT, EGR, and CPC. All authors approved the final version of the manuscript for submission.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
Ethics approval was obtained from the National-Healthcare Group Domain-Specific Review Board. The study was conducted in accordance with the Declaration of Helsinki.
Informed consent
Written informed consent was obtained in the preferred language of the participants or their legal representatives by bilingual study coordinators prior to their recruitment into the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Neurology
Electronic supplementary material
ESM 1
(DOC 253 kb)
Rights and permissions
About this article
Cite this article
Tanaka, T., Stephenson, M.C., Nai, YH. et al. Improved quantification of amyloid burden and associated biomarker cut-off points: results from the first amyloid Singaporean cohort with overlapping cerebrovascular disease. Eur J Nucl Med Mol Imaging 47, 319–331 (2020). https://doi.org/10.1007/s00259-019-04642-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-019-04642-8