Abstract
Rationale
Dopamine transporter (DAT) imaging is an important adjunct in the diagnostic workup of patients with Parkinsonism. 18F-FE-PE2I is a suitable PET radioligand for DAT quantification and imaging with good pharmacokinetics. The aim of this study was to determine a clinical optimal simplified reference tissue-based image acquisition protocol and to compare the discriminatory value and effect size for 18F-FE-PE2I to that for 123I-FP-CIT scan currently used in clinical practice.
Methods
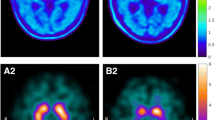
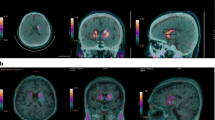
Nine patients with early Parkinson’s disease (PD, 64.3 ± 6.8 years, 3M), who had previously undergone a 123I-FP-CIT scan as part of their diagnostic workup, and 34 healthy volunteers (HV, 47.7 ± 16.8 years, 13M) underwent a 60-min dynamic 18F-FE-PE2I PET-MR scan on a GE Signa 3T PET-MR. Based on dynamic data and MR-based VOI delineation, BPND, semi-quantitative uptake ratio and SUVR[t1–t2] images were calculated using either occipital cortex or cerebellum as reference region. For start-and-end time of the SUVR interval, three time frames [t1–t2] were investigated: [15–40] min, [40–60] min, and [50–60] min postinjection. Data for putamen (PUT) and caudate nucleus-putamen ratio (CPR) were compared in terms of quantification bias versus BPND and discriminative power.
Results
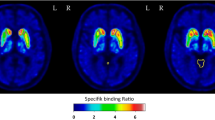
Using occipital cortex as reference region resulted in smaller bias of SUVR with respect to BPND + 1 and higher correlation between SUVR and BPND + 1 compared with using cerebellum, irrespective of SUVR [t1–t2] interval. Smallest bias was observed with the [15–40]-min time window, in accordance with previous literature. The correlation between BPND + 1 and SUVR was slightly better for the late time windows. Discriminant analysis between PD and HV using both PUT and CPR SUVRs showed an accuracy of ≥ 90%, for both reference regions and all studied time windows. Semi-quantitative 123I-FP-CIT and 18F-FE-PE2I values and relative decrease in the striatum for patients were highly correlated, with a higher effect size for 18F-FE-PE2I for PUT and CPR SUVR.
Conclusion
18F-FE-PE2I is a suitable radioligand for in vivo DAT imaging with high discriminative power between early PD and healthy controls. Whereas a [15–40]-min window has lowest bias with respect to BPND, a [50–60]-min time window at pseudoequilibrium can be advocated in terms of clinical feasibility with optimal discriminative power. The occipital cortex may be slightly preferable as reference region because of the higher time stability, stronger correlation of SUVR with BPND + 1, and lower bias. Moreover, the data suggest that the diagnostic accuracy of a 10-min static 18F-FE-PE2I scan is non-inferior compared with 123I-FP-CIT scan used in standard clinical practice.










Similar content being viewed by others
References
Fazio P, Svenningsson P, Cselenyi Z, Halldin C, Farde L, Varrone A. Nigrostriatal dopamine transporter availability in early Parkinson’s disease. Mov Disord. 2018;33(4):592–9.
Uhl GR. Dopamine transporter: basic science and human variation of a key molecule for dopaminergic function, locomotion, and Parkinsonism. Mov Disord. 2003;18(Suppl 7):S71–80.
Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J, et al. Parkinson disease. Nat Rev Dis Primers. 2017;3:17013.
Brooks DJ. Molecular imaging of dopamine transporters. Ageing Res Rev. 2016;30:114–21.
Jakobson Mo S, Axelsson J, Jonasson L, Larsson A, Ogren MJ, Ogren M, et al. Dopamine transporter imaging with [(18)F]FE-PE2I PET and [(123)I]FP-CIT SPECT-a clinical comparison. EJNMMI Res. 2018;8(1):100.
Rahmim A, Zaidi H. PET versus SPECT: strengths, limitations and challenges. Nucl Med Commun. 2008;29(3):193–207.
Sasaki T, Ito H, Kimura Y, Arakawa R, Takano H, Seki C, et al. Quantification of dopamine transporter in human brain using PET with 18F-FE-PE2I. J Nucl Med. 2012;53(7):1065–73.
Varrone A, Halldin C. New developments of dopaminergic imaging in Parkinson’s disease. Q J Nucl Med Mol Imaging. 2012;56(1):68–82.
Varrone A, Toth M, Steiger C, Takano A, Guilloteau D, Ichise M, et al. Kinetic analysis and quantification of the dopamine transporter in the nonhuman primate brain with 11C-PE2I and 18F-FE-PE2I. J Nucl Med. 2010;52(1):132–9.
Fazio P, Svenningsson P, Forsberg A, Jonsson EG, Amini N, Nakao R, et al. Quantitative analysis of (1)(8)F-(E)-N-(3-iodoprop-2-enyl)-2beta-carbofluoroethoxy-3beta-(4′-methyl-phenyl) nortropane binding to the dopamine transporter in Parkinson disease. J Nucl Med. 2015;56(5):714–20.
Pinborg LH, Ziebell M, Frokjaer VG, de Nijs R, Svarer C, Haugbol S, et al. Quantification of 123I-PE2I binding to dopamine transporter with SPECT after bolus and bolus/infusion. J Nucl Med. 2005;46(7):1119–27.
Ikoma Y, Sasaki T, Kimura Y, Seki C, Okubo Y, Suhara T, et al. Evaluation of semi-quantitative method for quantification of dopamine transporter in human PET study with (1)(8)F-FE-PE2I. Ann Nucl Med. 2015;29(8):697–708.
Sonni I, Fazio P, Schain M, Halldin C, Svenningsson P, Farde L, et al. Optimal acquisition time window and simplified quantification of dopamine transporter availability using 18F-FE-PE2I in healthy controls and Parkinson disease patients. J Nucl Med. 2016;57(10):1529–34.
Odano I, Varrone A, Hosoya T, Sakaguchi K, Gulyas B, Padmanabhan P, et al. Simplified estimation of binding parameters based on image-derived reference tissue models for dopamine transporter bindings in nonhuman primates using [(18)F]FE-PE2I and PET. Am J Nucl Med Mol Imaging. 2017;7(6):246–54.
Vermeulen RJ, Wolters EC, Tissingh G, Booij J, Janssen AG, Habraken J, et al. Evaluation of [123I] beta-CIT binding with SPECT in controls, early and late Parkinson’s disease. Nucl Med Biol. 1995;22(8):985–91.
Varrone A, Dickson JC, Tossici-Bolt L, Sera T, Asenbaum S, Booij J, et al. European multicentre database of healthy controls for [123I]FP-CIT SPECT (ENC-DAT): age-related effects, gender differences and evaluation of different methods of analysis. Eur J Nucl Med Mol Imaging. 2013;40(2):213–27.
Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord. 2010;25(15):2649–53.
Rezaei A, Schramm G, Van Laere K, Nuyts J. Estimation of crystal timing properties and efficiencies for the improvement of (joint) maximum-likelihood reconstructions in TOF-PET. IEEE Trans Med Imaging. 2019.
Schramm G, Koole M, Willekens S, Ahmadreza R, Van Weehaeghe D, Delso G et al. Regional accuracy of ZTE-based attenuation correction in static and dynamic brain PET/MR. Medical Physics. 2018.
Acknowledgments
The authors explicitly want to thank Mr. Kwinten Porters and Mr. Jef Van Loock for their contributions to the scanning and data handling, and the PET radiopharmacy and medical physics teams of UZ Leuven for their skilled contributions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Koen Van Laere and Wim Vandenberghe are Senior Clinical Investigators of the Fund for Scientific Research, Flanders, Belgium (FWO). Donatienne Van Weehaeghe and Aline Delva are PhD fellows of the FWO. Jenny Ceccarini is a postdoctoral fellow of the FWO. There are no other conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (UZ/KU Leuven Ethical Committee Belgium) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Neurology
Rights and permissions
About this article
Cite this article
Delva, A., Van Weehaeghe, D., van Aalst, J. et al. Quantification and discriminative power of 18F-FE-PE2I PET in patients with Parkinson’s disease. Eur J Nucl Med Mol Imaging 47, 1913–1926 (2020). https://doi.org/10.1007/s00259-019-04587-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-019-04587-y