Abstract
Purpose
Little is known regarding the clinical relevance or neurobiology of subtle motor disturbance in Alzheimer’s disease (AD). This study aims to investigate the patterns of striatal 18F-FP-CIT uptake in patients with AD-related cognitive impairment (ADCI) with mild parkinsonism.
Methods
We recruited 29 consecutive patients with ADCI with mild parkinsonism. All patients underwent 18F-FP-CIT PET scans and dopamine transporter (DAT) availability in striatal subregions (anterior/posterior caudate, anterior/posterior putamen, ventral putamen, ventral striatum) was quantified. Additionally, 32 patients with dementia with Lewy bodies (DLB) and 21 healthy controls were included to perform inter-group comparative analyses of the striatal DAT availability. The discriminatory power of striatal DAT availability to differentiate ADCI from DLB was assessed using receiver operating characteristics (ROC) analyses. The Spearman’s correlation coefficient was calculated to assess the relationship between motor severity and DAT availability in striatal subregions.
Results
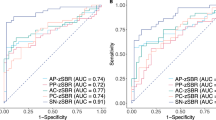
Patients with ADCI with mild parkinsonism exhibited decreased DAT availability in the caudate that was intermediate between healthy controls and patients with DLB. The DAT availability in other striatal subregions, including the posterior putamen, did not differ between the ADCI with parkinsonism and healthy control groups. The ROC analysis showed that DAT availability of all striatal subregions, especially the whole striatum, had a fair discriminatory power. Parkinsonian motor severity did not correlate with the striatal DAT availability in ADCI with parkinsonism.
Conclusions
The present study demonstrated that patients with ADCI with mild parkinsonism had distinct DAT scan patterns and suggests that parkinsonism is associated with the extranigral source of pathology.


Similar content being viewed by others
References
Horvath J, Burkhard PR, Herrmann FR, Bouras C, Kovari E. Neuropathology of parkinsonism in patients with pure Alzheimer’s disease. J Alzheimers Dis. 2014;39(1):115–20. https://doi.org/10.3233/jad-131289.
Molsa PK, Marttila RJ, Rinne UK. Extrapyramidal signs in Alzheimer’s disease. Neurology. 1984;34(8):1114–6.
Scarmeas N, Hadjigeorgiou GM, Papadimitriou A, Dubois B, Sarazin M, Brandt J, et al. Motor signs during the course of Alzheimer disease. Neurology. 2004;63(6):975–82.
Portet F, Scarmeas N, Cosentino S, Helzner EP, Stern Y. Extrapyramidal signs before and after diagnosis of incident Alzheimer disease in a prospective population study. Arch Neurol. 2009;66(9):1120–6. https://doi.org/10.1001/archneurol.2009.196.
Stern Y, Albert M, Brandt J, Jacobs DM, Tang MX, Marder K, et al. Utility of extrapyramidal signs and psychosis as predictors of cognitive and functional decline, nursing home admission, and death in Alzheimer’s disease: prospective analyses from the predictors study. Neurology. 1994;44(12):2300–7.
Reinikainen KJ, Paljarvi L, Halonen T, Malminen O, Kosma VM, Laakso M, et al. Dopaminergic system and monoamine oxidase-B activity in Alzheimer’s disease. Neurobiol Aging. 1988;9(3):245–52.
de la Monte SM, Wells SE, Hedley-Whyte T, Growdon JH. Neuropathological distinction between Parkinson’s dementia and Parkinson’s plus Alzheimer’s disease. Ann Neurol. 1989;26(3):309–20. https://doi.org/10.1002/ana.410260302.
Burns JM, Galvin JE, Roe CM, Morris JC, McKeel DW. The pathology of the substantia nigra in Alzheimer disease with extrapyramidal signs. Neurology. 2005;64(8):1397–403. https://doi.org/10.1212/01.wnl.0000158423.05224.7f.
Morris JC, Drazner M, Fulling K, Grant EA, Goldring J. Clinical and pathological aspects of parkinsonism in Alzheimer’s disease. A role for extranigral factors? Arch Neurol. 1989;46(6):651–7.
Braak H, Braak E. Alzheimer’s disease: striatal amyloid deposits and neurofibrillary changes. J Neuropathol Exp Neurol. 1990;49(3):215–24.
Murray AM, Weihmueller FB, Marshall JF, Hurtig HI, Gottleib GL, Joyce JN. Damage to dopamine systems differs between Parkinson’s disease and Alzheimer’s disease with parkinsonism. Ann Neurol. 1995;37(3):300–12. https://doi.org/10.1002/ana.410370306.
Schauer TH, Lochner M, Kovacs GG. Nigral tau pathology and striatal amyloid-beta deposition does not correlate with striatal dopamine deficit in Alzheimer’s disease. J Neural Transm (Vienna). 2012;119(12):1545–9. https://doi.org/10.1007/s00702-012-0832-9.
Rinne JO, Sahlberg N, Ruottinen H, Nagren K, Lehikoinen P. Striatal uptake of the dopamine reuptake ligand [11C]beta-CFT is reduced in Alzheimer’s disease assessed by positron emission tomography. Neurology. 1998;50(1):152–6.
Ceravolo R, Volterrani D, Gambaccini G, Bernardini S, Rossi C, Logi C, et al. Presynaptic nigro-striatal function in a group of Alzheimer’s disease patients with parkinsonism: evidence from a dopamine transporter imaging study. J Neural Transm (Vienna). 2004;111(8):1065–73. https://doi.org/10.1007/s00702-004-0140-0.
McKeith I, O’Brien J, Walker Z, Tatsch K, Booij J, Darcourt J, et al. Sensitivity and specificity of dopamine transporter imaging with 123I-FP-CIT SPECT in dementia with Lewy bodies: a phase III, multicentre study. Lancet Neurol. 2007;6(4):305–13. https://doi.org/10.1016/s1474-4422(07)70057-1.
Yoo HS, Jeon S, Chung SJ, Yun M, Lee PH, Sohn YH, et al. Olfactory dysfunction in Alzheimer’s disease- and Lewy body-related cognitive impairment. Alzheimers Dement. 2018. https://doi.org/10.1016/j.jalz.2018.05.010.
Landau SM, Harvey D, Madison CM, Koeppe RA, Reiman EM, Foster NL, et al. Associations between cognitive, functional, and FDG-PET measures of decline in AD and MCI. Neurobiol Aging. 2011;32(7):1207–18. https://doi.org/10.1016/j.neurobiolaging.2009.07.002.
Mistur R, Mosconi L, Santi SD, Guzman M, Li Y, Tsui W, et al. Current challenges for the early detection of Alzheimer’s disease: brain imaging and CSF studies. J Clin Neurol. 2009;5(4):153–66. https://doi.org/10.3988/jcn.2009.5.4.153.
Buchman AS, Wilson RS, Shulman JM, Leurgans SE, Schneider JA, Bennett DA. Parkinsonism in older adults and its association with adverse health outcomes and neuropathology. J Gerontol A Biol Sci Med Sci. 2016;71(4):549–56. https://doi.org/10.1093/gerona/glv153.
Buchman AS, Leurgans SE, Yu L, Wilson RS, Lim AS, James BD, et al. Incident parkinsonism in older adults without Parkinson disease. Neurology. 2016;87(10):1036–44. https://doi.org/10.1212/wnl.0000000000003059.
Chu Y, Buchman AS, Olanow CW, Kordower JH. Do subjects with minimal motor features have prodromal Parkinson disease? Ann Neurol. 2018;83(3):562–74. https://doi.org/10.1002/ana.25179.
McKeith IG, Boeve BF, Dickson DW, Halliday G, Taylor JP, Weintraub D, et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB consortium. Neurology. 2017;89(1):88–100. https://doi.org/10.1212/wnl.0000000000004058.
Blanc F, Colloby SJ, Cretin B, de Sousa PL, Demuynck C, O’Brien JT, et al. Grey matter atrophy in prodromal stage of dementia with Lewy bodies and Alzheimer’s disease. Alzheimers Res Ther. 2016;8:31. https://doi.org/10.1186/s13195-016-0198-6.
Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55(3):181–4.
Chung SJ, Yoo HS, Moon H, Oh JS, Kim JS, Park YH, et al. Early-onset drug-induced parkinsonism after exposure to offenders implies nigrostriatal dopaminergic dysfunction. J Neurol Neurosurg Psychiatry. 2018;89(2):169–74. https://doi.org/10.1136/jnnp-2017-315873.
Noh Y, Lee Y, Seo SW, Jeong JH, Choi SH, Back JH, et al. A new classification system for ischemia using a combination of deep and periventricular white matter hyperintensities. J Stroke Cerebrovasc Dis. 2014;23(4):636–42. https://doi.org/10.1016/j.jstrokecerebrovasdis.2013.06.002.
Ahn HJ, Chin J, Park A, Lee BH, Suh MK, Seo SW, et al. Seoul neuropsychological screening battery-dementia version (SNSB-D): a useful tool for assessing and monitoring cognitive impairments in dementia patients. J Korean Med Sci. 2010;25(7):1071–6. https://doi.org/10.3346/jkms.2010.25.7.1071.
Brooks DJ, Ibanez V, Sawle GV, Quinn N, Lees AJ, Mathias CJ, et al. Differing patterns of striatal 18F-dopa uptake in Parkinson’s disease, multiple system atrophy, and progressive supranuclear palsy. Ann Neurol. 1990;28(4):547–55. https://doi.org/10.1002/ana.410280412.
Gibb WR, Mountjoy CQ, Mann DM, Lees AJ. The substantia nigra and ventral tegmental area in Alzheimer’s disease and Down’s syndrome. J Neurol Neurosurg Psychiatry. 1989;52(2):193–200.
Liu Y, Stern Y, Chun MR, Jacobs DM, Yau P, Goldman JE. Pathological correlates of extrapyramidal signs in Alzheimer’s disease. Ann Neurol. 1997;41(3):368–74. https://doi.org/10.1002/ana.410410312.
Joyce JN, Smutzer G, Whitty CJ, Myers A, Bannon MJ. Differential modification of dopamine transporter and tyrosine hydroxylase mRNAs in midbrain of subjects with Parkinson’s, Alzheimer’s with parkinsonism, and Alzheimer’s disease. Mov Disord. 1997;12(6):885–97. https://doi.org/10.1002/mds.870120609.
Wolf DS, Gearing M, Snowdon DA, Mori H, Markesbery WR, Mirra SS. Progression of regional neuropathology in Alzheimer disease and normal elderly: findings from the Nun study. Alzheimer Dis Assoc Disord. 1999;13(4):226–31.
Almeida OP, Burton EJ, McKeith I, Gholkar A, Burn D, O’Brien JT. MRI study of caudate nucleus volume in Parkinson’s disease with and without dementia with Lewy bodies and Alzheimer’s disease. Dement Geriatr Cogn Disord. 2003;16(2):57–63. https://doi.org/10.1159/000070676.
Molina V, Lubeiro A, Blanco J, Blanco JA, Rodriguez M, Rodriguez-Campos A, et al. Parkinsonism is associated to fronto-caudate disconnectivity and cognition in schizophrenia. Psychiatry Res Neuroimaging. 2018;277:1–6. https://doi.org/10.1016/j.pscychresns.2018.04.009.
Cummings JL, Henchcliffe C, Schaier S, Simuni T, Waxman A, Kemp P. The role of dopaminergic imaging in patients with symptoms of dopaminergic system neurodegeneration. Brain. 2011;134(Pt 11):3146–66. https://doi.org/10.1093/brain/awr177.
Shimizu S, Namioka N, Hirose D, Kanetaka H, Hirao K, Hatanaka H, et al. Comparison of diagnostic utility of semi-quantitative analysis for DAT-SPECT for distinguishing DLB from AD. J Neurol Sci. 2017;377:50–4. https://doi.org/10.1016/j.jns.2017.03.040.
Jellinger KA, Attems J. Does striatal pathology distinguish Parkinson disease with dementia and dementia with Lewy bodies? Acta Neuropathol. 2006;112(3):253–60. https://doi.org/10.1007/s00401-006-0088-2.
Halliday GM, Song YJ, Harding AJ. Striatal beta-amyloid in dementia with Lewy bodies but not Parkinson’s disease. J Neural Transm (Vienna). 2011;118(5):713–9. https://doi.org/10.1007/s00702-011-0641-6.
Brilliant MJ, Elble RJ, Ghobrial M, Struble RG. The distribution of amyloid beta protein deposition in the corpus striatum of patients with Alzheimer’s disease. Neuropathol Appl Neurobiol. 1997;23(4):322–5.
O’Brien JT, Colloby S, Fenwick J, Williams ED, Firbank M, Burn D, et al. Dopamine transporter loss visualized with FP-CIT SPECT in the differential diagnosis of dementia with Lewy bodies. Arch Neurol. 2004;61(6):919–25. https://doi.org/10.1001/archneur.61.6.919.
Walker Z, Costa DC, Walker RW, Lee L, Livingston G, Jaros E, et al. Striatal dopamine transporter in dementia with Lewy bodies and Parkinson disease: a comparison. Neurology. 2004;62(9):1568–72.
Joling M, Vriend C, van der Zande JJ, Lemstra AW, van den Heuvel OA, Booij J, et al. Lower (123)I-FP-CIT binding to the striatal dopamine transporter, but not to the extrastriatal serotonin transporter, in Parkinson’s disease compared with dementia with Lewy bodies. NeuroImage Clinical. 2018;19:130–6. https://doi.org/10.1016/j.nicl.2018.04.009.
Chung SJ, Yoo HS, Oh JS, Kim JS, Ye BS, Sohn YH, et al. Effect of striatal dopamine depletion on cognition in de novo Parkinson’s disease. Parkinsonism Relat Disord. 2018;51:43–8. https://doi.org/10.1016/j.parkreldis.2018.02.048.
Roselli F, Pisciotta NM, Perneczky R, Pennelli M, Aniello MS, De Caro MF, et al. Severity of neuropsychiatric symptoms and dopamine transporter levels in dementia with Lewy bodies: a 123I-FP-CIT SPECT study. Mov Disord. 2009;24(14):2097–103. https://doi.org/10.1002/mds.22702.
Ziebell M, Andersen BB, Pinborg LH, Knudsen GM, Stokholm J, Thomsen G, et al. Striatal dopamine transporter binding does not correlate with clinical severity in dementia with Lewy bodies. J Nucl Med. 2013;54(7):1072–6. https://doi.org/10.2967/jnumed.112.114025
Siepel FJ, Dalen I, Gruner R, Booij J, Bronnick KS, Buter TC, et al. Loss of dopamine transporter binding and clinical symptoms in dementia with Lewy bodies. Mov Disord. 2016;31(1):118–25. https://doi.org/10.1002/mds.26327.
Shimizu S, Hirao K, Kanetaka H, Namioka N, Hatanaka H, Hirose D, et al. Utility of the combination of DAT SPECT and MIBG myocardial scintigraphy in differentiating dementia with Lewy bodies from Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2016;43(1):184–92. https://doi.org/10.1007/s00259-015-3146-y.
Ravina B, Eidelberg D, Ahlskog JE, Albin RL, Brooks DJ, Carbon M, et al. The role of radiotracer imaging in Parkinson disease. Neurology. 2005;64(2):208–15. https://doi.org/10.1212/01.wnl.0000149403.14458.7f.
Funding
This research was supported by a grant from the Korea Health Technology R&D Project through the Korean Healthy Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI16C1118).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 192 kb)
Rights and permissions
About this article
Cite this article
Chung, S.J., Lee, Y.H., Yoo, H.S. et al. Distinct FP-CIT PET patterns of Alzheimer’s disease with parkinsonism and dementia with Lewy bodies. Eur J Nucl Med Mol Imaging 46, 1652–1660 (2019). https://doi.org/10.1007/s00259-019-04315-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-019-04315-6