Abstract
Purpose
18F-Fluorodeoxyglucose positron emission tomography (18F-FDG PET/CT) is frequently used to diagnose fracture-related infections (FRIs), but its diagnostic performance in this field is still unknown. The aims of this study were: (1) to assess the diagnostic performance of qualitative assessment of 18F-FDG PET/CT scans in diagnosing FRI, (2) to establish the diagnostic performance of standardized uptake values (SUVs) extracted from 18F-FDG PET/CT scans and to determine their associated optimal cut-off values, and (3) to identify variables that predict a false-positive (FP) or false-negative (FN) 18F-FDG PET/CT result.
Methods
This retrospective cohort study included all patients with suspected FRI undergoing 18F-FDG PET/CT between 2011 and 2017 in two level-1 trauma centres. Two nuclear medicine physicians independently reassessed all 18F-FDG PET/CT scans. The reference standard consisted of the result of at least two deep, representative microbiological cultures or the presence/absence of clinical confirmatory signs of FRI (AO/EBJIS consensus definition) during a follow-up of at least 6 months. Diagnostic performance in terms of sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) was calculated. Additionally, SUVs were measured on 18F-FDG PET/CT scans. Volumes of interest were drawn around the suspected and corresponding contralateral areas to obtain absolute values and ratios between suspected and contralateral areas. A multivariable logistic regression analysis was also performed to identify the most important predictor(s) of FP or FN 18F-FDG PET/CT results.
Results
The study included 156 18F-FDG PET/CT scans in 135 patients. Qualitative assessment of 18F-FDG PET/CT scans showed a sensitivity of 0.89, specificity of 0.80, PPV of 0.74, NPV of 0.91 and diagnostic accuracy of 0.83. SUVs on their own resulted in lower diagnostic performance, but combining them with qualitative assessments yielded an AUC of 0.89 compared to an AUC of 0.84 when considering only the qualitative assessment results (p = 0.007). 18F-FDG PET/CT performed <1 month after surgery was found to be the independent variable with the highest predictive value for a false test result, with an absolute risk of 46% (95% CI 27–66%), compared with 7% (95% CI 4–12%) in patients with 18F-FDG PET/CT performed 1–6 months after surgery.
Conclusion
Qualitative assessment of 18F-FDG PET/CT scans had a diagnostic accuracy of 0.83 and an excellent NPV of 0.91 in diagnosing FRI. Adding SUV measurements to qualitative assessment provided additional accuracy in comparison to qualitative assessment alone. An interval between surgery and 18F-FDG PET/CT of <1 month was associated with a sharp increase in false test results.
Similar content being viewed by others
Introduction
Fracture-related infection (FRI) is a serious complication following trauma surgery and can lead to increased morbidity and high medical costs [1, 2]. Clinical symptoms are not always evident, therefore diagnosing FRI can be challenging. This problem was worsened by the fact that, until recently, there was no uniform definition of FRI [3]. Recently, the AO Foundation (Arbeitsgemeinschaft für Osteosynthesefragen) and the European Bone and Joint Infection Society (EBJIS) published a consensus definition comprising confirmatory and suggestive criteria for diagnosing FRI [4]. Medical imaging is considered to be only an adjunct to the diagnosis of FRI (i.e. a suggestive criterion). The reason for this is that the evidence for its accuracy in diagnosing FRI is limited. Moreover, such evidence as is available was obtained mainly from studies investigating other causes of bone infection such as diabetic foot infection, periprosthetic joint infection (PJI) and haematogenous osteomyelitis [5]. Most previous studies on diagnostic imaging of FRI have been hampered by small patient cohorts, unclear reference standards and heterogeneous patient populations [5, 6]. Recently, our group found that white blood cell (WBC) scintigraphy has a high accuracy (0.92) when diagnosing FRI [7]. To compare imaging modalities, we used the same study design to evaluate the diagnostic performance of 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT).
The aims of the current study were:
-
1.
To establish the performance of qualitative assessment of 18F-FDG PET/CT scans in diagnosing FRI
-
2)
To establish the performance of standardized uptake values (SUVs) from 18F-FDG PET/CT in diagnosing FRI and to determine their optimal associated cut-off values
-
3)
To determine which variables are independent predictors of a false positive (FP) or false negative (FN) 18F-FDG PET/CT test result in patients with suspected FRI
Materials and methods
Ethical approval
Due to the observational nature of this study the need for informed consent was waived by the Medical Ethics Review Committee (METC) of the University Medical Center Utrecht (METC 17-475).
Study design and eligibility criteria
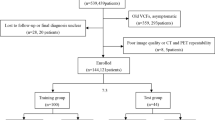
This was a two-centre, retrospective cohort study that included patients from two large level-1 trauma centres in The Netherlands: the University Medical Center Utrecht and the University Medical Center Groningen. All consecutive patients undergoing 18F-FDG PET/CT for diagnosing (or excluding) FRI between January 2011 and November 2017 were eligible for inclusion. FRI was considered as either an infection following an open fracture (irrespective of type of treatment), an infection following fracture surgery, or an infection following instrumented fusion for spinal fractures. Skeletally immature patients (<16 years old) and patients undergoing 18F-FDG PET/CT for reasons other than diagnosing FRI (such as PJI, nontraumatic osteosyntheses or haematogenous osteomyelitis) were excluded. Patients in whom the reference test did not meet the criteria for validity, as described in the section Reference test, were also excluded.
Index test
The index test was the 18F-FDG PET/CT scan. Scanning protocols were similar in both centres. Scans were acquired approximately 60 min after intravenous administration of 2–3 MBq/kg 18F-FDG according to existing European Association of Nuclear Medicine (EANM) guidelines for 18F imaging [8]. Scans were acquired on either a Biograph mCT 64-slice or a Biograph mCT 40-slice PET/CT system (Siemens, Knoxville, TN, USA). No metal artefact reduction algorithm was used in either centre.
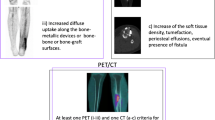
After anonymization, the scans were independently reassessed by two experienced nuclear medicine physicians (M.G.G.H. and A.W.J.M.G.). Both the attenuation-corrected images and the images without attenuation correction were reviewed. Both nuclear medicine physicians were blinded to the reference test result. Nuclear imaging signs were documented for each of the scans on a case report form (CRF). These signs included uptake location, uptake pattern (multifocal, heterogeneous, diffuse homogeneous), uptake grade (0: no uptake, 1: higher uptake in the side with suspected infection than in the contralateral side, 2: much higher uptake in the side with suspected infection than in the contralateral side), involvement of osteosynthesis material, and soft-tissue and bone involvement. Disagreements were resolved through discussion until consensus was reached. A clinical case example of the use of 18F-FDG PET/CT for diagnosing FRI is provided in Fig. 1.
A 59-year-old man sustained a right-sided Gustilo grade IIIB open crural fracture (a) which was treated with intramedullary nailing and a fasciotomy (b). After several soft-tissue debridement procedures, the remaining soft tissue defect was eventually closed with a free musculocutaneous flap. After 20 months, there was a non-union with “autodynamization” of the intramedullary nail, demonstrated by broken interlocking screws (c). The 18F-FDG PET image (d) shows increased uptake around the fracture site in the tibial shaft and around the proximal and distal screws. The hybrid 18F-FDG PET/CT images (e axial, f coronal, g sagittal) localize the suspected fracture-related infection (FRI) not only to the fracture site but also to the surrounding bone of the tibia around the fracture site which corresponds to the unstable scar overlapping the area of the non-union (h). The intramedullary nail was removed, the tibia was reamed, the fracture site was debrided and an in-house, custom-made antibiotic nail was inserted (I). FRI was confirmed by microbiological cultures and the patient was subsequently treated with antibiotics. One year after exchange nailing, fracture healing was successful (j)
For semiquantitative analysis, SUVs were also measured on 18F-FDG-PET/CT scans reconstructed according to EANM EARL protocols. SUVs correspond to the extent of 18F-FDG uptake and consequently reflect cellular glucose metabolism. Because glucose metabolism is increased in infected tissues, higher SUVs correspond to a greater risk of FRI than lower SUVs [9]. SUVs were determined by drawing a spherical volume of interest (VOI) on both the target area with suspected infection and a corresponding anatomical reference area on the contralateral side. Additionally, a VOI was drawn on nearby muscle for background comparison. For all VOIs, both SUVmax (single-pixel value) and SUVpeak (average value in a high-uptake part of the VOI) were calculated. For both SUVmax and SUVpeak, the ratios between the suspected infected side and the contralateral side were also calculated (SUVmaxratio and SUVpeakratio). To correct for background 18F-FDG uptake, ratios between the SUVs of the suspected infected site and the SUVs of nearby muscles (SUVmaxmuscleratio and SUVpeakmuscleratio) were calculated. These data were reported in a separate CRF as continuous measurements. All SUV measurements were corrected for body weight and blood glucose level and were performed with syngo.via software (Siemens Healthineers, Forchheim, Germany).
Reference test
The final diagnosis of FRI (reference test) was based on the outcome of medical microbiological (MMB) culture results in patients with surgical intervention, or – if unavailable – on clinical follow-up of at least 6 months. Because this study involved the retrospective analysis of culture results obtained in an era when no uniform culturing protocol existed, strict criteria for judging the validity of the reference test were applied. All MMB results were judged by an experienced trauma surgeon on their ability to correctly detect FRI. The microbiological results from swabs and cultures of fistulas were disregarded due to relatively low accuracy [10,11,12]. The MMB results were only considered representative if cultures of at least two surgically obtained deep-tissue samples from the site of suspected infection were available. A positive FRI result was defined as at least two positive representative MMB cultures with the same microorganism according to the microbiological criteria outlined in the AO/EBJIS consensus definition [4]. FRI during clinical follow-up was defined according to the clinical confirmatory criteria of the AO/EBJIS consensus definition as any wound breakdown, purulent drainage or the presence or development of a sinus tract (communicating with the implant material) [4]. If culture results were negative but confirmatory criteria for FRI were met (e.g. pus, fistula) peroperatively when cultures were taken, FRI was deemed to be present (and the culture result was considered to be erroneous). Culture-negative FRIs are known to be caused by bacteria with low virulence such as coagulase-negative Staphylococcus species [13].
Statistical analyses
To assess the diagnostic performance of the 18F-FDG PET/CT scan, the number of true-positive (TP), FP, true-negative (TN) and FN test results were obtained. From this, sensitivity, specificity, positive predictive values (PPV), negative predictive values (NPV), positive and negative likelihood ratios and diagnostic odds ratios with 95% confidence intervals (CI) were calculated. A sensitivity analysis was performed including only the first scan in each patient to determine whether selection bias of patients undergoing multiple scans may have contributed to differences in diagnostic parameters.
All SUVs were compared between groups using Student’s t test (if normally distributed) or the Mann-Whitney U test (if not normally distributed). Normality of the data was determined by visual inspection of normality plots. The sensitivity and specificity of the separate SUV measurements were plotted as receiver operating characteristic (ROC) curves and for each curve, the area under the curve (AUC) was calculated. The Q-point on each curve (i.e. the point at which sensitivity and specificity were maximized) was determined and the associated cut-off value was extracted. In addition, an ROC curve was plotted combining the diagnostic performance of SUV measurements with the performance of qualitative assessment. The difference between the ROC curve from the combined analysis and the ROC curve with only the qualitative assessment was analysed using the test described by DeLong et al. [14]. To ensure that this test was appropriately applied in this situation of nested models, we investigated whether the added variable “combined SUV measurements” in the combined model was independently associated with the outcome [15].
Consequently, a backward stepwise multivariable logistic regression analysis was performed to determine which variables were independent predictors of a false (i.e. FP or FN) test result. Removal testing was performed with the probabilities of the likelihood ratio statistic based on the maximum partial likelihood estimates. Multiple variables suggested in the literature to influence 18F-FDG PET/CT accuracy were included in the model [16]. The variables entered were: interval between the last operative procedure (or date of trauma if no operation was performed) and the 18F-FDG PET/CT scan (ordinal; <1 month, between 1 and 6 months and >6 months), body mass index (continuous), presence of diabetes mellitus (dichotomous), smoking history (dichotomous), nonsteroidal antiinflammatory drug (NSAID) use at the time of 18F-FDG PET/CT (dichotomous) and antibiotic use at the time of 18F-FDG PET/CT (dichotomous). Using the final model, the probabilities of false test results were obtained (with 95% CIs) for the different variables. Additionally, the diagnostic performance of qualitative assessment was calculated excluding scans with a high risk of a false test result. All statistical analyses were performed with SPSS Statistics version 25.0 (IBM Corp., Armonk, NY).
Results
In the study period, 154 patients underwent 176 18F-FDG PET/CT scans for suspected FRI. The reference test was not performed in 18 patients and these patients were excluded. Two 18F-FDG PET/CT scans in skeletally immature patients were also excluded. A total of 135 patients who underwent 156 18F-FDG PET/CT scans were ultimately included. The patient characteristics are summarized in Table 1. The fracture specifics are presented in Table 2, and the types of index operation in Table 3.
For 67 18F-FDG PET/CT scans (43%), a representative MMB culture result was available. These scans were obtained from patients with a median clinical follow-up of 13 months (IQR 20 months), 33 of these scans (49%) were obtained from patients that had a MMB culture-confirmed FRI. Staphylococcus species were most commonly cultured (Table 4). In 11 patients, culture results were negative but there were peroperative confirmatory signs of FRI, including purulent drainage, wound breakdown or a fistula communicating with implant material. These patients were scored as positive for FRI.
For 89 18F-FDG PET/CT scans (57%), representative MMB culture results were not available. These scans were obtained from patients with a median clinical follow-up of 16 months (IQR 23 months), 18 of these scans were obtained from patients that showed clinical confirmatory signs of FRI, the remainder of these patients had an uneventful clinical follow-up. The 71 remaining patients had an uneventful clinical follow-up. In total, 62 patients were diagnosed with FRI. In 55 of these 62 patients, 18F-FDG PET/CT was positive for FRI (TP). In 75 of 94 patients negative for FRI, 18F-FDG PET/CT correctly ruled out an FRI (TN). The 18F-FDG PET/CT result was FP in 19 patients and FN in 7 patients. Thus, 18F-FDG PET/CT showed a diagnostic sensitivity of 0.89 (95% CI 0.78–0.95), specificity of 0.80 (95% CI 0.70–0.87), PPV of 0.74 (95% CI 0.66–0.81), NPV of 0.91 (95% CI 0.84–0.96), positive likelihood ratio of 4.39 (95% CI 2.91–6.62), negative likelihood ratio of 0.14 (95% CI 0.07–0.29), and diagnostic odds ratio of 31.0 (95% CI 12.2–78.9). The accuracy of 18F-FDG PET/CT for diagnosing FRI was 0.83 (95% CI 0.77–0.89). The sensitivity analysis including only the first 18F-FDG PET/CT scan in each patient (N = 135) resulted in similar diagnostic parameters: sensitivity 0.91 (95% CI 0.80–0.97), specificity 0.81 (95% CI 0.70–0.89), PPV 0.77 (95% CI 0.68–0.84), NPV 0.93 (95% CI 0.84–0.97) and diagnostic accuracy 0.85 (95% CI 0.78–0.91).
Semiquantitative measurements
Semiquantitative SUV measurements are presented in Table 5. Patients with FRI had a median SUVmax of 5.9 (IQR 3.5) and median SUVpeak of 4.7 (IQR 2.4) in the area with suspected infection. Patients without FRI had a median SUVmax of 3.2 (IQR 2.5) and a median SUVpeak of 2.6 (IQR 1.9) in the area initially suspected of infection. The differences in both SUVmax and SUVpeak between the groups were significant (both p < 0.001). In patients with FRI, the SUV ratios for the area with suspected infection in relation to the contralateral area were 3.0 (IQR 2.1) for SUVmax and 2.9 (IQR 2.0) for SUVpeak. In patients without FRI, the ratios were 1.9 (IQR 1.4) and 1.8 (IQR 1.4), respectively. Both ratios were significantly different between patients with and without FRI (p < 0.001). In patients with FRI, the SUV ratios for the area with suspected infection in relation to nearby muscle were 6.4 (IQR 4.9) for SUVmax and 5.5 (IQR 3.6) for SUVpeak. In patients without FRI, the ratios were 3.5 (IQR 3.0) and 3.3 (IQR 2.9), respectively. These ratios were also significantly different between patients with and without FRI (p < 0.001).
ROC curves for the semiquantitative SUV data are shown in Fig. 2. The areas under the curve were 0.80 (95% CI 0.73–0.88) for SUVmax, 0.73 (95% CI 0.64–0.81) for SUVmaxratio and 0.77 (95% CI 0.70–0.85) for SUVmaxmuscleratio. Optimal sensitivity and specificity for SUVmax were 0.80 and 0.72 at a cut-off value of 4.2. The PPV and NPV for SUVmax at this cut-off value were 0.65 and 0.85, respectively. For SUVmaxratio, sensitivity was 0.75 and specificity was 0.62 at a cut-off value of 2.0, and for SUVmaxmuscleratio, sensitivity was 0.74 and specificity was 0.68 at a cut-off value of 4.7. The diagnostic parameters and associated cut-off values for SUVpeak were similar to those for SUVmax and are also shown in Fig. 2.
Receiver operating characteristics (ROC) curves for the semiquantitative SUV measurements analysed separately and in combination with the qualitative 18F-FDG PET/CT assessment data. The circles on the curves represent the Q-points (i.e. the optimum between sensitivity and specificity at a specific cut-off value). The cross represents the sensitivity and specificity of the qualitative 18F-FDG PET/CT assessment. This point is higher than any of the Q-points for the semiquantitative measurements alone. The area under the curve for the combined qualitative and semiquantitative assessment (dotted line) is 0.89, higher than the areas under the curve for the semiquantitative measurements analysed separately and also higher than the AUC of the qualitative assessment alone. AUROC area under the receiver operator characteristics curve, SN sensitivity, SP specificity, PPV positive predictive value, NPV negative predictive value
Combining the SUV measurement data with the qualitative assessment of 18F-FDG PET/CT scans in a separate ROC curve yielded an AUC of 0.89 (95% CI 0.84–0.95) and a diagnostic accuracy of 0.86 (sensitivity 0.85, specificity 0.87, PPV 0.81, NPV 0.90), in contrast to an AUC of 0.84 (95% CI 0.78–0.91) and a diagnostic accuracy of 0.83 for the qualitative assessment on its own. The added explanatory variable “combined SUV measurements” was independently associated with the presence/absence of FRI and comparison of the ROC curves was deemed appropriate. The AUC of the combined assessment was 0.05 (95% CI 0.01–0.09) greater than the AUC of the qualitative assessment alone (p = 0.007).
Characteristics of patients with false-negative/false-positive results
Seven patients were included with a FN test result. Two patients had positive intraoperative cultures, while five patients showed confirmatory signs peroperatively or during the 6-month follow-up. Two patients had (low-grade) infection of a non-union (both ankle fractures). Another patient (with two scans) showed peroperative signs of FRI in the tibia (infected tissue and pus) despite microbiological cultures remaining negative. There were 19 patients with a FP test result. These included two patients with a lower arm fracture, two with a femoral fracture, two with a tibial plateau fracture, seven with a lower leg fracture, two with an ankle fracture, two with a talar fracture and two with a spinal fracture. Eight patients had a negative intraoperative culture, 11 had no cultures taken but showed no signs of FRI during the 6-month follow-up. Five patients (26%) with a FP result underwent surgery during the week before the 18F-FDG PET/CT scan (one with a tibial fracture, one with a talar fracture, one with an ankle fracture, and two with a tibial plateau fracture). These scans were performed to determine if the FRI had receded or was still advancing in patients who underwent surgery for suspected FRI shortly before the scan.
Predictors of a false test result
The most important predictor of a false test result was an interval of <1 month between the last operative procedure and the 18F-FDG PET/CT scan (B = 2.461, intercept −2.615). The associated absolute predicted risk of a false result with this variable was 46% (95% CI 27–66%) compared with an absolute predicted risk of the reference group (with an interval of 1–6 months) of 7% (95% CI 4–12%). In patients with an interval of >6 months, the absolute risk was 17% (95% CI 10–29%). The test result was erroneous in 6 of 14 patients (42.9%) undergoing 18F-FDG PET/CT within 1 month (FP in all six patients). The rate of erroneous test results reduced to 8.9% (4 of 45 patients) in those with an interval between 1 and 6 months, and showed a slight increase to 16.8% (16 out of 95 patients) in those with an interval of more than 6 months. Omitting the results from the early 18F-FDG PET/CT scans (performed within 1 month of surgery) led to an increase in diagnostic accuracy of the qualitative assessment to 0.86 (95% CI 0.79–0.91) with a sensitivity and specificity of 0.88 (95% CI 0.76–0.95) and 0.85 (95% CI 0.76–0.92), respectively.
Discussion
The current study showed that qualitative assessment of 18FDG PET/CT scans has good performance in diagnosing FRI with a diagnostic accuracy of 0.83 (95% CI 0.77–0.89) and an AUC of 0.84 (95% CI 0.78–0.91). The NPV (0.91) was notably higher than that of most other imaging modalities, and makes 18FDG PET/CT an excellent tool for use in patients with chronic or low-grade infections [5]. Combining the results of qualitative assessment and SUV measurements resulted in an even higher diagnostic accuracy (0.86) and an AUC of 0.89 (95% CI 0.84–0.95), which shows that including SUV measurements increased diagnostic accuracy, although the increase was relatively small.
The sensitivity and specificity rates found in this study are in line with those found in other studies on the accuracy of 18F-FDG PET/CT in diagnosing FRI [5, 9]. However, this study also included semiquantitative measurements and used strict 18F-FDG PET/CT assessment and reference test criteria (based on the recently released AO/EBJIS consensus definition of FRI) [4]. It also included the largest series to date of patients with suspected FRI undergoing hybrid 18F-FDG PET/CT imaging. One systematic review and meta-analysis investigating the accuracy of different imaging modalities for diagnosing chronic osteomyelitis showed higher diagnostic accuracy of 18F-FDG PET with a pooled sensitivity of 0.96 and a specificity of 0.91 [6]. That study, however, included only studies published before 2003 and investigated only 18F-FDG PET without fusion CT images, which is now rarely used following the advent of 18F-FDG PET/CT scanners. In addition, reference test criteria were unclear in some of the studies reviewed and the studies included few patients and a relatively large number of spinal 18F-FDG PET/CT scans. A more recent systematic review found that the sensitivities and specificities of 18F-FDG-PET/CT in diagnosing FRI ranges between 0.86–0.94 and 0.76–1.00, respectively [5]. These results, as well as the methodology used (patient population and reference standard) are comparable to those used in our study.
There is only limited research on the accuracy of quantification in diagnosing FRI. A recent study on the accuracy of SUV measurements from 18F-FDG PET/CT for diagnosing FRI found a sensitivity of 0.65 and specificity of 0.77 at a SUVmax cut-off value of 4.0 [17]. These values are lower than those published previously for qualitative assessment of 18F-FDG PET/CT scans [5]. The reason for this could be that the previous SUV measurement study used only 18F-FDG PET/CT to differentiate between infected non-unions and aseptic non-unions. In both circumstances, increased bone metabolism will often be found, and thus differences between 18F-FDG uptake will be limited. The cut-off value of 4.0 used in the previous study is similar to the SUVmax cut-off value found in the current study (4.2). Unfortunately, the validity of the results is difficult to compare between our study and the previous study, because it is unclear whether the standardized EARL scanning protocols were used in the latter [18]. Additionally, only semiquantitative measurements, and no qualitative criteria (such as uptake pattern and grade) for diagnosing FRI were used. SUV measurements do not take into account the activity pattern and uptake location, and can be positive as a consequence of both bone healing and/or non-union. Therefore, using only semiquantitative data might lead to misclassification of some patients. This is supported by the results of our study, in which the diagnostic accuracy of the qualitative assessment by the nuclear medicine physicians was higher than the accuracy when using SUVs alone. This phenomenon was also seen in a large study of patients with FRI which demonstrated a diagnostic accuracy of 0.82 with qualitative assessment of 18F-FDG PET(/CT) scans and a lower accuracy with only semiquantitative measurements (SUVmax sensitivity 0.69, specificity 0.66 using a cut-off value of 3.9) [9]. Another study investigating SUVs in histologically proven culture-positive and culture-negative patients with FRI showed that SUVs in both groups of patients were similar (SUVmax 3.73 in culture-positive patients, 2.81 in culture-negative patients) [19]. The findings of these studies, as well as those of the current study, add to the mounting evidence that semiquantitative measurements can be used as additional diagnostic tools for diagnosing FRI.
WBC scintigraphy has been more thoroughly investigated as an imaging modality for diagnosing FRI. Our previous study of WBC scintigraphy found a diagnostic accuracy of 0.92, which is higher than the diagnostic accuracy found in the current study for 18F-FDG PET/CT [7]. However, 18F-FDG PET/CT does have several advantages over WBC scintigraphy. First, there is no need for manipulation of leukocytes, which is a labourious and expensive part of WBC scintigraphy [20]. Second, 18F-FDG PET/CT can be performed much more quickly (1 h following radionuclide injection) and takes only one scanning session, as opposed to WBC scintigraphy, which takes at least two scans (4 h and 20–24 h after radionuclide injection) on two consecutive days [20]. Third, WBC scintigraphy has lower accuracy when used for diagnosing infections in the axial skeleton due to physiological uptake in the bone marrow, while 18F-FDG PET/CT does not have this limitation [16]. 18F-FDG PET/CT has the disadvantage that implants negatively affect diagnostic accuracy, although in some studies, this effect has not been shown [5, 9]. With the recent onset of several techniques for metal artefact reduction in the newest generation PET/CT camera systems, the diagnostic performance of both qualitative assessment and quantification in patients with an implant and suspected FRI can probably be improved further. Ultimately, both imaging modalities have their specific advantages and limitations and although 18F-FDG PET/CT has lower accuracy than WBC scintigraphy, its advantages in terms of logistics and patient comfort make it a good alternative to WBC scintigraphy as the first nuclear imaging modality to perform when diagnosing FRI. Thus, both modalities can be used to diagnose FRI depending on physician/hospital preference, financial considerations, and/or experience with either technique.
We found that performing the 18F-FDG PET/CT scan <1 month following surgery was correlated with a FP 18F-FDG PET/CT result. It is known that operative procedures cause tissue damage and inflammation/regeneration, and affected tissue shows increased uptake of 18F-FDG, especially when the interval between the 18F-FDG PET/CT and surgery is short [16]. Five of the FP 18F-FDG PET/CT scans were performed within a week of an operative procedure. Both nuclear medicine physicians reassessing these scans for this study agreed that in some of these scans, inflammation due to surgery was indistinguishable from FRI. We conclude that 18F-FDG PET/CT should therefore not be performed as a diagnostic tool within a month of surgery. If (per protocol) early (<1 month after surgery) 18F-FDG PET/CT scans for suspected FRI are no longer performed, diagnostic accuracy can be expected to improve, in this study exclusion of such early scans led to an increase in accuracy from 0.83 to 0.86.
The strengths of the current study are the large cohort size, and the fact that a robust, standardized and repeatable scan assessment was performed by two independent nuclear medicine physicians (one from each hospital) who were blinded to the reference standard. We also used strict reference standard criteria to determine whether FRI was present or not, based on the recently published FRI consensus definition [4]. Finally, the addition of SUV measurements and SUV analysis provided additional insight into its merits and its performance compared to standard qualitative assessments.
The limitations of the current study include its retrospective design, with the associated risks of selection- and differential misclassification bias. Patients were recruited in two different teaching hospitals, thus there may have been differences in the diagnostic work-up and treatment of FRI, as each hospital has its own standard of care. Also, in some patients, FRI had already been diagnosed and the 18F-FDG PET/CT scans were used for treatment follow-up. This mainly occurred at the beginning of the study period; since then, stricter protocols have been adopted, which aim to standardize both 18F-FDG PET/CT indications and microbiological culture acquisition and treatment regimens. Finally, it is important to remember that the combined assessment by two nuclear medicine specialists might have led to a higher diagnostic accuracy than can be obtained in the normal clinical situation, in which only one nuclear medicine physician reviews a scan. Further prospective studies to compare different imaging modalities for diagnosing FRI are warranted.
Conclusion
The results of the study can be summarized as follows:
-
1.
Qualitative assessment of 18F-FDG PET/CT scans has good accuracy (0.83) for diagnosing FRI, with an excellent NPV of 0.91.
-
2.
SUV measurements provide additional diagnostic accuracy when added to qualitative assessment of 18F-FDG PET/CT scans.
-
3.
18F-FDG PET/CT should not be performed for diagnosis within a month of surgery.
References
Metsemakers WJ, Smeets B, Nijs S, Hoekstra H. Infection after fracture fixation of the tibia: analysis of healthcare utilization and related costs. Injury. 2017;48(6):1204–10. https://doi.org/10.1016/j.injury.2017.03.030.
Whitehouse JD, Friedman ND, Kirkland KB, Richardson WJ, Sexton DJ. The impact of surgical-site infections following orthopedic surgery at a community hospital and a university hospital: adverse quality of life, excess length of stay, and extra cost. Infect Control Hosp Epidemiol. 2002;23(4):183–9. https://doi.org/10.1086/502033.
Metsemakers WJ, Kortram K, Morgenstern M, Moriarty TF, Meex I, Kuehl R, et al. Definition of infection after fracture fixation: a systematic review of randomized controlled trials to evaluate current practice. Injury. 2018;49(3):497–504. https://doi.org/10.1016/j.injury.2017.02.010.
Metsemakers WJ, Morgenstern M, McNally MA, Moriarty TF, McFadyen I, Scarborough M, et al. Fracture-related infection: a consensus on definition from an international expert group. Injury. 2018;49(3):505–10. https://doi.org/10.1016/j.injury.2017.08.040.
Govaert GA, IJpma FFA, McNally M, McNally E, Reininga IH, Glaudemans AW. Accuracy of diagnostic imaging modalities for peripheral post-traumatic osteomyelitis – a systematic review of the recent literature. Eur J Nucl Med Mol Imaging. 2017;44(8):1393–407. https://doi.org/10.1007/s00259-017-3683-7.
Termaat MF, Raijmakers PG, Scholten HJ, Bakker FC, Patka P, Haarman HJ. The accuracy of diagnostic imaging for the assessment of chronic osteomyelitis: a systematic review and meta-analysis. J Bone Joint Surg Am. 2005;87(11):2464–71. https://doi.org/10.2106/JBJS.D.02691.
Govaert GAM, Bosch P, IJpma FFA, Glauche J, Jutte PC, Lemans JVC, et al. High diagnostic accuracy of white blood cell scintigraphy for fracture related infections: results of a large retrospective single-center study. Injury. 2018;49(6):1085–90. https://doi.org/10.1016/j.injury.2018.03.018.
Boellaard R, Delgado-Bolton R, Oyen WJ, Giammarile F, Tatsch K, Eschner W, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2015;42(2):328–54. https://doi.org/10.1007/s00259-014-2961-x.
Wenter V, Muller JP, Albert NL, Lehner S, Fendler WP, Bartenstein P, et al. The diagnostic value of [(18)F] FDG PET for the detection of chronic osteomyelitis and implant-associated infection. Eur J Nucl Med Mol Imaging. 2016;43(4):749–61. https://doi.org/10.1007/s00259-015-3221-4.
Aggarwal VK, Higuera C, Deirmengian G, Parvizi J, Austin MS. Swab cultures are not as effective as tissue cultures for diagnosis of periprosthetic joint infection. Clin Orthop Relat Res. 2013;471(10):3196–203.
Omar M, Suero EM, Liodakis E, Reichling M, Guenther D, Decker S, et al. Diagnostic performance of swab PCR as an alternative to tissue culture methods for diagnosing infections associated with fracture fixation devices. Injury. 2016;47(7):1421–6. https://doi.org/10.1016/j.injury.2016.04.038.
Zuluaga AF, Galvis W, Jaimes F, Vesga O. Lack of microbiological concordance between bone and non-bone specimens in chronic osteomyelitis: an observational study. BMC Infect Dis. 2002;2:8.
Morgenstern C, Cabric S, Perka C, Trampuz A, Renz N. Synovial fluid multiplex PCR is superior to culture for detection of low-virulent pathogens causing periprosthetic joint infection. Diagn Microbiol Infect Dis. 2018;90(2):115–9. https://doi.org/10.1016/j.diagmicrobio.2017.10.016.
DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–45.
Demler OV, Pencina MJ, D’Agostino RB Sr. Misuse of DeLong test to compare AUCs for nested models. Stat Med. 2012;31(23):2577–87.
Glaudemans AW, Israel O, Slart RH. Pitfalls and limitations of radionuclide and hybrid imaging in infection and inflammation. Semin Nucl Med. 2015;45(6):500–12. https://doi.org/10.1053/j.semnuclmed.2015.02.005.
van Vliet KE, de Jong VM, Termaat MF, Schepers T, van Eck-Smit BLF, Goslings JC, et al. FDG-PET/CT for differentiating between aseptic and septic delayed union in the lower extremity. Arch Orthop Trauma Surg. 2018;138(2):189–94. https://doi.org/10.1007/s00402-017-2806-8.
Kaalep A, Sera T, Oyen W, Krause BJ, Chiti A, Liu Y, et al. EANM/EARL FDG-PET/CT accreditation – summary results from the first 200 accredited imaging systems. Eur J Nucl Med Mol Imaging. 2018;45(3):412–22. https://doi.org/10.1007/s00259-017-3853-7.
Lankinen P, Seppanen M, Mattila K, Kallajoki M, Knuuti J, Aro HT. Intensity of (18)F-FDG PET uptake in culture-negative and culture-positive cases of chronic osteomyelitis. Contrast Media Mol Imaging. 2017;2017:9754293. https://doi.org/10.1155/2017/9754293.
Glaudemans AW, de Vries EF, Vermeulen LE, Slart RH, Dierckx RA, Signore A. A large retrospective single-centre study to define the best image acquisition protocols and interpretation criteria for white blood cell scintigraphy with 99mTc-HMPAO-labelled leucocytes in musculoskeletal infections. Eur J Nucl Med Mol Imaging. 2013;40(11):1760–9. https://doi.org/10.1007/s00259-013-2481-0.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the principles of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Due to the observational nature of this study the need for informed consent was waived by the Medical Ethics Review Committee (METC) of the University Medical Center Utrecht (METC 17-475).
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Lemans, J.V.C., Hobbelink, M.G.G., IJpma, F.F.A. et al. The diagnostic accuracy of 18F-FDG PET/CT in diagnosing fracture-related infections. Eur J Nucl Med Mol Imaging 46, 999–1008 (2019). https://doi.org/10.1007/s00259-018-4218-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-018-4218-6