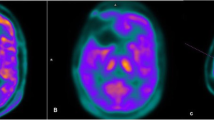
Abstract
Introduction
Radiological assessment of brain tumors is widely based on the Radiology Assessment of Neuro-Oncology (RANO) criteria that consider non-specific T1 and T2 weighted images. Limitation of the RANO criteria is that they do not include metabolic imaging techniques that have been reported to be helpful to differentiate treatment related changes from true tumor progression. In the current study, we assessed if the combined use of MRI and PET with hybrid 11C–MET PET/MRI can improve diagnostic accuracy and diagnostic confidence of the readers to differentiate treatment related changes from true progression in recurrent glioma.
Methods
Fifty consecutive patients with histopathologically proven glioma were prospectively enrolled for a hybrid 11C–MET PET/MRI to differentiate recurrent glioma from treatment induced changes. Sole MRI data were analyzed based on RANO. Sole PET data and in a third evaluation hybrid 11C–MET-PET/MRI data were assessed for metabolic respectively metabolic and morphologic glioma recurrence. Diagnostic performance and diagnostic confidence of the reader were calculated for the different modalities, and the McNemar test and Mann-Whitney U Test were applied for statistical analysis.
Results
Hybrid 11C–MET PET/MRI was successfully performed in all 50 patients. Glioma recurrence was diagnosed in 35 of the 50 patients (70%). Sensitivity and specificity were calculated for MRI (86.11% and 71.43%), for 11C–MET PET (96.77% and 73.68%), and for hybrid 11C–MET-PET/MRI (97.14% and 93.33%). For diagnostic accuracy hybrid 11C–MET-PET/MRI (96%) showed significantly higher values than MRI alone (82%), whereas no significant difference was found for 11C–MET PET (88%). Furthermore, by rating on a five-point Likert scale significantly higher scores were found for diagnostic confidence when comparing 11C–MET PET/MRI (4.26 ± 0,777) to either PET alone (3.44 ± 0.705) or MRI alone (3.56 ± 0.733).
Conclusion
This feasibility study showed that hybrid PET/MRI might strengthen RANO classification by adding metabolic information to conventional MRI information. Future studies should evaluate the clinical utility of the combined use of 11C–MET PET/MRI in larger patient cohorts.


Similar content being viewed by others
References
Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol : Off J Am Soc Clin Oncol. 2010;28:1963–72. https://doi.org/10.1200/JCO.2009.26.3541.
Radbruch A, Lutz K, Wiestler B, Baumer P, Heiland S, Wick W, et al. Relevance of T2 signal changes in the assessment of progression of glioblastoma according to the response assessment in Neurooncology criteria. Neuro-Oncology. 2012;14:222–9. https://doi.org/10.1093/neuonc/nor200.
Mullins ME, Barest GD, Schaefer PW, Hochberg FH, Gonzalez RG, Lev MH. Radiation necrosis versus glioma recurrence: conventional MR imaging clues to diagnosis. AJNR Am J Neuroradiol. 2005;26:1967–72.
Chamberlain MC, Glantz MJ, Chalmers L, Van Horn A, Sloan AE. Early necrosis following concurrent Temodar and radiotherapy in patients with glioblastoma. J Neuro-Oncol. 2007;82:81–3. https://doi.org/10.1007/s11060-006-9241-y.
Sorensen AG, Batchelor TT, Wen PY, Zhang WT, Jain RK. Response criteria for glioma. Nat Clin Pract Oncol. 2008;5:634–44. https://doi.org/10.1038/ncponc1204.
Stuplich M, Hadizadeh DR, Kuchelmeister K, Scorzin J, Filss C, Langen KJ, et al. Late and prolonged pseudoprogression in glioblastoma after treatment with lomustine and temozolomide. J Clin Oncol : Off J Am Soc Clin Oncol. 2012;30:e180–3. https://doi.org/10.1200/JCO.2011.40.9565.
Shah AH, Snelling B, Bregy A, Patel PR, Tememe D, Bhatia R, et al. Discriminating radiation necrosis from tumor progression in gliomas: a systematic review what is the best imaging modality? J Neuro-Oncol. 2013;112:141–52. https://doi.org/10.1007/s11060-013-1059-9.
Galldiks N, Dunkl V, Stoffels G, Hutterer M, Rapp M, Sabel M, et al. Diagnosis of pseudoprogression in patients with glioblastoma using O-(2-[18F]fluoroethyl)-L-tyrosine PET. Eur J Nucl Med Mol Imaging. 2015;42:685–95. https://doi.org/10.1007/s00259-014-2959-4.
Radbruch A, Fladt J, Kickingereder P, Wiestler B, Nowosielski M, Baumer P, et al. Pseudoprogression in patients with glioblastoma: clinical relevance despite low incidence. Neuro-Oncology. 2015;17:151–9. https://doi.org/10.1093/neuonc/nou129.
Brandsma D, Stalpers L, Taal W, Sminia P, van den Bent MJ. Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol. 2008;9:453–61. https://doi.org/10.1016/S1470-2045(08)70125-6.
Blasel S, Zagorcic A, Jurcoane A, Bahr O, Wagner M, Harter PN, et al. Perfusion MRI in the evaluation of suspected Glioblastoma recurrence. J Neuroimaging. 2016;26:116–23. https://doi.org/10.1111/jon.12247.
Seeger A, Braun C, Skardelly M, Paulsen F, Schittenhelm J, Ernemann U, et al. Comparison of three different MR perfusion techniques and MR spectroscopy for multiparametric assessment in distinguishing recurrent high-grade gliomas from stable disease. Acad Radiol. 2013;20:1557–65. https://doi.org/10.1016/j.acra.2013.09.003.
Kazda T, Bulik M, Pospisil P, Lakomy R, Smrcka M, Slampa P, et al. Advanced MRI increases the diagnostic accuracy of recurrent glioblastoma: single institution thresholds and validation of MR spectroscopy and diffusion weighted MR imaging. Neuroimage Clin. 2016;11:316–21. https://doi.org/10.1016/j.nicl.2016.02.016.
Bobek-Billewicz B, Stasik-Pres G, Majchrzak H, Zarudzki L. Differentiation between brain tumor recurrence and radiation injury using perfusion, diffusion-weighted imaging and MR spectroscopy. Folia Neuropathol. 2010;48:81–92.
Hu LS, Baxter LC, Smith KA, Feuerstein BG, Karis JP, Eschbacher JM, et al. Relative cerebral blood volume values to differentiate high-grade glioma recurrence from posttreatment radiation effect: direct correlation between image-guided tissue histopathology and localized dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging measurements. AJNR Am J Neuroradiol. 2009;30:552–8. https://doi.org/10.3174/ajnr.A1377.
Hamstra DA, Galban CJ, Meyer CR, Johnson TD, Sundgren PC, Tsien C, et al. Functional diffusion map as an early imaging biomarker for high-grade glioma: correlation with conventional radiologic response and overall survival. J Clin Oncol : Off J Am Soc Clin Oncol. 2008;26:3387–94. https://doi.org/10.1200/JCO.2007.15.2363.
Kebir S, Fimmers R, Galldiks N, Schafer N, Mack F, Schaub C, et al. Late Pseudoprogression in Glioblastoma: diagnostic value of dynamic O-(2-[18F]fluoroethyl)-L-tyrosine PET. Clin Cancer Res. 2016;22:2190–6. https://doi.org/10.1158/1078-0432.CCR-15-1334.
Galldiks N, Langen KJ. Amino acid PET - an imaging option to identify treatment response, Posttherapeutic effects, and tumor recurrence? Front Neurol. 2016;7:120. https://doi.org/10.3389/fneur.2016.00120.
Herholz K, Langen KJ, Schiepers C, Mountz JM. Brain tumors. Semin Nucl Med. 2012;42:356–70. https://doi.org/10.1053/j.semnuclmed.2012.06.001.
Herholz K, Holzer T, Bauer B, Schroder R, Voges J, Ernestus RI, et al. 11C-methionine PET for differential diagnosis of low-grade gliomas. Neurology. 1998;50:1316–22.
Langstrom B, Antoni G, Gullberg P, Halldin C, Malmborg P, Nagren K, et al. Synthesis of L- and D-[methyl-11C]methionine. J Nucl Med : Off Publ, Soc Nucl Med. 1987;28:1037–40.
Schober O, Duden C, Meyer GJ, Muller JA, Hundeshagen H. Non selective transport of [11C-methyl]-L-and D-methionine into a malignant glioma. Eur J Nucl Med. 1987;13:103–5.
Tsuyuguchi N, Takami T, Sunada I, Iwai Y, Yamanaka K, Tanaka K, et al. Methionine positron emission tomography for differentiation of recurrent brain tumor and radiation necrosis after stereotactic radiosurgery--in malignant glioma. Ann Nucl Med. 2004;18:291–6.
Li DL, Xu YK, Wang QS, Wu HB, Li HS. (1)(1)C-methionine and (1)(8)F-fluorodeoxyglucose positron emission tomography/CT in the evaluation of patients with suspected primary and residual/recurrent gliomas. Chin Med J (Engl). 2012;125:91–6.
Yamane T, Sakamoto S, Senda M. Clinical impact of (11)C-methionine PET on expected management of patients with brain neoplasm. Eur J Nucl Med Mol Imaging. 2010;37:685–90. https://doi.org/10.1007/s00259-009-1302-y.
Galldiks N, Ullrich R, Schroeter M, Fink GR, Jacobs AH, Kracht LW. Volumetry of [(11)C]-methionine PET uptake and MRI contrast enhancement in patients with recurrent glioblastoma multiforme. Eur J Nucl Med Mol Imaging. 2010;37:84–92. https://doi.org/10.1007/s00259-009-1219-5.
Albert NL, Weller M, Suchorska B, Galldiks N, Soffietti R, Kim MM, et al. Response assessment in Neuro-oncology working group and European Association for Neuro-Oncology recommendations for the clinical use of PET imaging in gliomas. Neuro-Oncology. 2016;18:1199–208. https://doi.org/10.1093/neuonc/now058.
Delso G, Furst S, Jakoby B, Ladebeck R, Ganter C, Nekolla SG, et al. Performance measurements of the Siemens mMR integrated whole-body PET/MR scanner. J Clin Oncol : Off J Am Soc Clin Oncol. 2011;52:1914–22. https://doi.org/10.2967/jnumed.111.092726.
Quick HH. Integrated PET/MR. J Magnet Resonan imaging : JMRI. 2014;39:243–58. https://doi.org/10.1002/jmri.24523.
Deuschl C, Goericke S, Grueneisen J, Sawicki LM, Goebel J, El Hindy N, et al. Simultaneous 11C-Methionine positron emission tomography/magnetic resonance imaging of suspected primary brain Tumors. PLoS One. 2016;11:e0167596. https://doi.org/10.1371/journal.pone.0167596.
Rapp M, Heinzel A, Galldiks N, Stoffels G, Felsberg J, Ewelt C, et al. Diagnostic performance of 18F-FET PET in newly diagnosed cerebral lesions suggestive of glioma. J Clin Oncol : Off J Am Soc Clin Oncol. 2013;54:229–35. https://doi.org/10.2967/jnumed.112.109603.
Terakawa Y, Tsuyuguchi N, Iwai Y, Yamanaka K, Higashiyama S, Takami T, et al. Diagnostic accuracy of 11C-methionine PET for differentiation of recurrent brain tumors from radiation necrosis after radiotherapy. J Clin Oncol : Off J Am Soc Clin Oncol. 2008;49:694–9. https://doi.org/10.2967/jnumed.107.048082.
Tripathi M, Sharma R, Varshney R, Jaimini A, Jain J, Souza MM, et al. Comparison of F-18 FDG and C-11 methionine PET/CT for the evaluation of recurrent primary brain tumors. Clin Nucl Med. 2012;37:158–63. https://doi.org/10.1097/RLU.0b013e318238f51a.
Peca C, Pacelli R, Elefante A, Del Basso De Caro ML, Vergara P, Mariniello G, et al. Early clinical and neuroradiological worsening after radiotherapy and concomitant temozolomide in patients with glioblastoma: tumour progression or radionecrosis? Clin Neurol Neurosurg. 2009;111:331–4. https://doi.org/10.1016/j.clineuro.2008.11.003.
Hygino da Cruz LC Jr, Rodriguez I, Domingues RC, Gasparetto EL, Sorensen AG. Pseudoprogression and pseudoresponse: imaging challenges in the assessment of posttreatment glioma. AJNR American journal of neuroradiology. 2011;32:1978–85. https://doi.org/10.3174/ajnr.A2397.
Kreth FW, Muacevic A, Medele R, Bise K, Meyer T, Reulen HJ. The risk of haemorrhage after image guided stereotactic biopsy of intra-axial brain tumours--a prospective study. Acta Neurochir (Wien). 2001;143:539–45. discussion 45-6
Enslow MS, Zollinger LV, Morton KA, Butterfield RI, Kadrmas DJ, Christian PE, et al. Comparison of 18F-fluorodeoxyglucose and 18F-fluorothymidine PET in differentiating radiation necrosis from recurrent glioma. Clin Nucl Med. 2012;37:854–61. https://doi.org/10.1097/RLU.0b013e318262c76a.
Nozawa A, Rivandi AH, Kanematsu M, Hoshi H, Piccioni D, Kesari S, et al. Glucose-corrected standardized uptake value in the differentiation of high-grade glioma versus post-treatment changes. Nucl Med Commun. 2015;36:573–81. https://doi.org/10.1097/MNM.0000000000000288.
Garibotto V, Heinzer S, Vulliemoz S, Guignard R, Wissmeyer M, Seeck M, et al. Clinical applications of hybrid PET/MRI in neuroimaging. Clin Nucl Med. 2013;38:e13–8. https://doi.org/10.1097/RLU.0b013e3182638ea6.
Grosu AL, Astner ST, Riedel E, Nieder C, Wiedenmann N, Heinemann F, et al. An interindividual comparison of O-(2-[18F]fluoroethyl)-L-tyrosine (FET)- and L-[methyl-11C]methionine (MET)-PET in patients with brain gliomas and metastases. Int J Radiat Oncol Biol Phys. 2011;81:1049–58. https://doi.org/10.1016/j.ijrobp.2010.07.002.
D'Souza MM, Sharma R, Jaimini A, Panwar P, Saw S, Kaur P, et al. 11C-MET PET/CT and advanced MRI in the evaluation of tumor recurrence in high-grade gliomas. Clin Nucl Med. 2014;39:791–8. https://doi.org/10.1097/RLU.0000000000000532.
Okamoto S, Shiga T, Hattori N, Kubo N, Takei T, Katoh N, et al. Semiquantitative analysis of C-11 methionine PET may distinguish brain tumor recurrence from radiation necrosis even in small lesions. Ann Nucl Med. 2011;25:213–20. https://doi.org/10.1007/s12149-010-0450-2.
Minamimoto R, Saginoya T, Kondo C, Tomura N, Ito K, Matsuo Y, et al. Differentiation of brain tumor recurrence from post-radiotherapy necrosis with 11C-Methionine PET: visual assessment versus quantitative assessment. PLoS One. 2015;10:e0132515. https://doi.org/10.1371/journal.pone.0132515.
Takano K, Kinoshita M, Arita H, Okita Y, Chiba Y, Kagawa N, et al. Diagnostic and prognostic value of 11C-Methionine PET for nonenhancing Gliomas. AJNR Am J Neuroradiol. 2016;37:44–50. https://doi.org/10.3174/ajnr.A4460.
Singhal T, Narayanan TK, Jacobs MP, Bal C, Mantil JC. 11C-methionine PET for grading and prognostication in gliomas: a comparison study with 18F-FDG PET and contrast enhancement on MRI. J Clin Oncol : Off J Am Soc Clin Oncol. 2012;53:1709–15. https://doi.org/10.2967/jnumed.111.102533.
Acknowledgements
We thank Christoph Ritter, MSc, for technical help in the statistical analysis.
Funding
An IFORES grant to CD from the University Duisburg-Essen supported the research (http://www.uni-due.de/med/forschung/forschungsfoerderung/ifores.shtml). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Ethical approval
All procedures performed were in accordance with the ethical standards of the institutional research committee and with the principles of the 1964 Declaration of Helsinki and its later amendments.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Deuschl, C., Kirchner, J., Poeppel, T.D. et al. 11C–MET PET/MRI for detection of recurrent glioma. Eur J Nucl Med Mol Imaging 45, 593–601 (2018). https://doi.org/10.1007/s00259-017-3916-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-017-3916-9