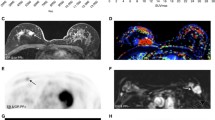
Abstract
Purpose
Imaging biomarkers from functional imaging modalities were assessed as potential surrogate markers of disease status. Specifically, in this prospective study, we investigated the relationships between functional imaging parameters and histological prognostic factors and breast cancer subtypes.
Methods
In total, 43 patients with large or locally advanced invasive ductal carcinoma (IDC) were analyzed (47.6 ± 7.5 years old). 68Ga-Labeled arginine-glycine-aspartic acid (RGD) and 18F-fluorodeoxyglucose (FDG) positron emission tomography/computed tomography (PET/CT) were performed. The maximum and average standardized uptake values (SUVmax and SUVavg) from RGD PET/CT and SUVmax and SUVavg from FDG PET/CT were the imaging parameters used. For histological prognostic factors, estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) expression was identified using immunohistochemistry (IHC) or fluorescent in situ hybridization (FISH). Four breast cancer subtypes, based on ER/PR and HER2 expression (ER/PR+,Her2−, ER/PR+,Her2+, ER/PR−,Her2+, and ER/PR−,Her2−), were considered.
Results
Quantitative FDG PET parameters were significantly higher in the ER-negative group (15.88 ± 8.73 vs 10.48 ± 6.01, p = 0.02 for SUVmax; 9.40 ± 5.19 vs 5.92 ± 4.09, p = 0.02 for SUVavg) and the PR-negative group (8.37 ± 4.94 vs 4.79 ± 3.93, p = 0.03 for SUVavg). Quantitative RGD PET parameters were significantly higher in the HER2-positive group (2.42 ± 0.59 vs 2.90 ± 0.75, p = 0.04 for SUVmax; 1.60 ± 0.38 vs 1.95 ± 0.53, p = 0.04 for SUVavg) and showed a significant positive correlation with the HER2/CEP17 ratio (r = 0.38, p = 0.03 for SUVmax and r = 0.46, p < 0.01 for SUVavg). FDG PET parameters showed significantly higher values in the ER/PR−,Her2− subgroup versus the ER/PR+,Her2− or ER/PR+,Her2+ subgroups, while RGD PET parameters showed significantly lower values in the ER/PR−,Her2− subgroup versus the other subgroups. There was no correlation between FDG and RGD PET parameters in the overall group. Only the ER/PR−,Her2− subgroup showed a significant positive correlation between FDG and RGD PET parameters (r = 0.59, p = 0.03 for SUVmax).
Conclusion
68Ga-RGD and 18F-FDG PET/CT are promising functional imaging modalities for predicting biomarkers and molecular phenotypes in breast cancer patients.





Similar content being viewed by others
References
Allred D, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol 1998;11:155–68.
Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA 2006;295:2492–502.
Nguyen PL, Taghian AG, Katz MS, Niemierko A, Abi Raad RF, Boon WL, et al. Breast cancer subtype approximated by estrogen receptor, progesterone receptor, and HER-2 is associated with local and distant recurrence after breast-conserving therapy. J Clin Oncol 2008;26:2373–8.
Onitilo AA, Engel JM, Greenlee RT, Mukesh BN. Breast cancer subtypes based on ER/PR and Her2 expression: comparison of clinicopathologic features and survival. Clin Med Res 2009;7:4–13.
Chen W, Delaloye S, Silverman DHS, Geist C, Czernin J, Sayre J, et al. Predicting treatment response of malignant gliomas to bevacizumab and irinotecan by imaging proliferation with [18F] fluorothymidine positron emission tomography: a pilot study. J Clin Oncol 2007;25:4714–21.
Willett CG, Duda DG, di Tomaso E, Boucher Y, Ancukiewicz M, Sahani DV, et al. Efficacy, safety, and biomarkers of neoadjuvant bevacizumab, radiation therapy, and fluorouracil in rectal cancer: a multidisciplinary phase II study. J Clin Oncol 2009;27:3020–6.
Beer AJ, Kessler H, Wester HJ, Schwaiger M. PET imaging of integrin αvβ3 expression. Theranostics 2011;1:48–57.
Weis SM, Cheresh DA. αv Integrins in angiogenesis and cancer. Cold Spring Harb Perspect Med 2011;1:a006478.
Arpino G, Bardou VJ, Clark GM, Elledge RM. Infiltrating lobular carcinoma of the breast: tumor characteristics and clinical outcome. Breast Cancer Res 2004;6:R149–56.
Kim JH, Lee JS, Kang KW, Lee HY, Han SW, Kim TY, et al. Whole-body distribution and radiation dosimetry of (68)Ga-NOTA-RGD, a positron emission tomography agent for angiogenesis imaging. Cancer Biother Radiopharm 2012;27:65–71.
Wolff AC, Hammond MEH, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch Pathol Lab Med 2007;131:18–43.
Beer AJ, Haubner R, Sarbia M, Goebel M, Luderschmidt S, Grosu AL, et al. Positron emission tomography using [18F]Galacto-RGD identifies the level of integrin αvβ3 expression in man. Clin Cancer Res 2006;12:3942–9.
Beer AJ, Grosu AL, Carlsen J, Kolk A, Sarbia M, Stangier I, et al. [18F]Galacto-RGD positron emission tomography for imaging of αvβ3 expression on the neovasculature in patients with squamous cell carcinoma of the head and neck. Clin Cancer Res 2007;13:6610–6.
Kim YH, Jeon J, Hong SH, Rhim WK, Lee YS, Youn H, et al. Tumor targeting and imaging using cyclic RGD-PEGylated gold nanoparticle probes with directly conjugated iodine-125. Small 2011;7:2052–60.
Zhu A, Shim H. Current molecular imaging positron emitting radiotracers in oncology. Nucl Med Mol Imaging 2011;45:1–14.
Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer 2010;10:9–22.
Brooks PC, Clark R, Cheresh DA. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science 1994;264:569–71.
Friedlander M, Brooks PC, Shaffer RW, Kincaid CM, Varner JA, Cheresh DA. Definition of two angiogenic pathways by distinct αv integrins. Science 1995;270:1500–2.
Ross JS, Fletcher JA, Linette GP, Stec J, Clark E, Ayers M, et al. The Her-2/neu gene and protein in breast cancer 2003: biomarker and target of therapy. Oncologist 2003;8:307–25.
Kumar R, Yarmand-Bagheri R. The role of HER2 in angiogenesis. Semin Oncol 2001;28:27–32.
Yen L, Benlimame N, Nie Z-R, Xiao D, Wang T, Al Moustafa A-E, et al. Differential regulation of tumor angiogenesis by distinct ErbB homo- and heterodimers. Mol Biol Cell 2002;13:4029–44.
Petit A, Rak J, Hung MC, Rockwell P, Goldstein N, Fendly B, et al. Neutralizing antibodies against epidermal growth factor and ErbB-2/neu receptor tyrosine kinases down-regulate vascular endothelial growth factor production by tumor cells in vitro and in vivo: angiogenic implications for signal transduction therapy of solid tumors. Am J Pathol 1997;151:1523–30.
Russell KS, Stern DF, Polverini PJ, Bender JR. Neuregulin activation of ErbB receptors in vascular endothelium leads to angiogenesis. Am J Physiol 1999;277:H2205–11.
Vameşu S. Angiogenesis and c-erbB-2 (HER2/neu) overexpression status in primary breast cancer patients: an analysis of 158 needle core biopsies. Rom J Morphol Embryol 2007;48:121–9.
Ellis C, Dyson M, Stephenson T, Maltby E. HER2 amplification status in breast cancer: a comparison between immunohistochemical staining and fluorescence in situ hybridisation using manual and automated quantitative image analysis scoring techniques. J Clin Pathol 2005;58:710–4.
Ellis I, Dowsett M, Bartlett J, Walker R, Cooke T, Gullick W, et al. Recommendations for HER2 testing in the UK. J Clin Pathol 2000;53:890–2.
Schnell O, Krebs B, Carlsen J, Miederer I, Goetz C, Goldbrunner RH, et al. Imaging of integrin αvβ3 expression in patients with malignant glioma by [18F] Galacto-RGD positron emission tomography. Neuro Oncol 2009;11:861–70.
Ueda S, Tsuda H, Asakawa H, Shigekawa T, Fukatsu K, Kondo N, et al. Clinicopathological and prognostic relevance of uptake level using 18F-fluorodeoxyglucose positron emission tomography/computed tomography fusion imaging (18F-FDG PET/CT) in primary breast cancer. Jpn J Clin Oncol 2008;38:250–8.
Kim BS, Sung SH. Usefulness of 18F-FDG uptake with clinicopathologic and immunohistochemical prognostic factors in breast cancer. Ann Nucl Med 2012;26:175–83.
Bosch A, Eroles P, Zaragoza R, Viña JR, Lluch A. Triple-negative breast cancer: molecular features, pathogenesis, treatment and current lines of research. Cancer Treat Rev 2010;36:206–15.
Groheux D, Giacchetti S, Moretti J-L, Porcher R, Espié M, Lehmann-Che J, et al. Correlation of high 18F-FDG uptake to clinical, pathological and biological prognostic factors in breast cancer. Eur J Nucl Med Mol Imaging 2011;38:426–35.
Koolen B, Vrancken Peeters MJ, Wesseling J, Lips E, Vogel W, Aukema T, et al. Association of primary tumour FDG uptake with clinical, histopathological and molecular characteristics in breast cancer patients scheduled for neoadjuvant chemotherapy. Eur J Nucl Med Mol Imaging 2012;39:1830–8.
Arpino G, Wiechmann L, Osborne CK, Schiff R. Crosstalk between the estrogen receptor and the HER tyrosine kinase receptor family: molecular mechanism and clinical implications for endocrine therapy resistance. Endocr Rev 2008;29:217–33.
Guo J, Higashi K, Ueda Y, Oguchi M, Takegami T, Toga H, et al. Microvessel density: correlation with 18F-FDG uptake and prognostic impact in lung adenocarcinomas. J Nucl Med 2006;47:419–25.
Bos R, van der Hoeven JJ, van der Wall E, van der Groep P, van Diest PJ, Comans EF, et al. Biologic correlates of (18)fluorodeoxyglucose uptake in human breast cancer measured by positron emission tomography. J Clin Oncol 2002;20:379–87.
Cherk MH, Foo SS, Poon AM, Knight SR, Murone C, Papenfuss AT, et al. Lack of correlation of hypoxic cell fraction and angiogenesis with glucose metabolic rate in non–small cell lung cancer assessed by 18F-fluoromisonidazole and 18F-FDG PET. J Nucl Med 2006;47:1921–6.
Avril N, Menzel M, Dose J, Schelling M, Weber W, Jänicke F, et al. Glucose metabolism of breast cancer assessed by 18F-FDG PET: histologic and immunohistochemical tissue analysis. J Nucl Med 2001;42:9–16.
Beer AJ, Lorenzen S, Metz S, Herrmann K, Watzlowik P, Wester H-J, et al. Comparison of integrin αvβ3 expression and glucose metabolism in primary and metastatic lesions in cancer patients: a PET study using 18F-galacto-RGD and 18F-FDG. J Nucl Med 2008;49:22–9.
Linderholm B, Hellborg H, Johansson U, Elmberger G, Skoog L, Lehtiö J, et al. Significantly higher levels of vascular endothelial growth factor (VEGF) and shorter survival times for patients with primary operable triple-negative breast cancer. Ann Oncol 2009;20:1639–46.
Cameron D, Brown J, Dent R, Jackisch C, Mackey J, Pivot X, et al. Adjuvant bevacizumab-containing therapy in triple-negative breast cancer (BEATRICE): primary results of a randomised, phase 3 trial. Lancet Oncol 2013;14:933–42.
Gerber B, Loibl S, Eidtmann H, Rezai M, Fasching P, Tesch H, et al. Neoadjuvant bevacizumab and anthracycline–taxane-based chemotherapy in 678 triple-negative primary breast cancers; results from the geparquinto study (GBG 44). Ann Oncol 2013;24:2978–84.
Janssen M, Oyen WJ, Massuger LF, Frielink C, Dijkgraaf I, Edwards DS, et al. Comparison of a monomeric and dimeric radiolabeled RGD-peptide for tumor targeting. Cancer Biother Radiopharm 2002;17:641–6.
Acknowledgement
This study was supported by a grant of the Korea Healthcare Technology R&D Project, Ministry for Health & Welfare, Republic of Korea (A070001 and A100716).
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Yoon, HJ., Kang, K.W., Chun, I.K. et al. Correlation of breast cancer subtypes, based on estrogen receptor, progesterone receptor, and HER2, with functional imaging parameters from 68Ga-RGD PET/CT and 18F-FDG PET/CT. Eur J Nucl Med Mol Imaging 41, 1534–1543 (2014). https://doi.org/10.1007/s00259-014-2744-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-014-2744-4