Abstract
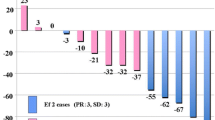
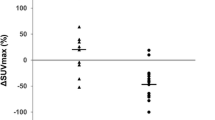
The purpose of this study was to clarify the clinical implications of thymic fluorodeoxyglucose (FDG) uptake after chemotherapy in pediatric patients with malignant disease. Twenty-two pediatric patients, aged 0–17 years (mean 7.0 years), who had undergone FDG positron emission tomography (PET) were examined retrospectively. A total of 48 FDG-PET scans of the 22 patients were reviewed. Seven PET scans were recorded before the initial chemotherapy, and the remaining 41 scans were conducted during and after chemotherapy. Thymic FDG uptake was evaluated using standardized uptake value (SUV) analysis. The effect of chemotherapy on thymic FDG uptake was assessed by comparing SUV before, during, and after chemotherapy. The change in thymic FDG uptake with increasing time after the completion of chemotherapy was also assessed. Clinical laboratory data including number of white blood cells (WBCs), erythrocytes (RBCs), platelets, plasma glucose level and differential white blood count were analyzed for their association with thymic FDG uptake. Thymic FDG uptake in patients during chemotherapy was significantly lower than that in patients before chemotherapy (mean±SD 1.71±0.48 vs 2.27±0.32, respectively, P<0.01). Thymic FDG uptake in patients after chemotherapy was significantly higher than that in patients during chemotherapy (mean±SD 2.74±0.70 vs 1.71±0.48, respectively, P<0.01). A significant relationship between thymic FDG uptake and interval after completion of chemotherapy (r=0.74, P<0.0001) was observed. Significant relationships between blood counts (WBCs, RBCs, and platelets) and thymic FDG uptake were also observed. Comparison of the differential white blood count and thymic FDG uptake revealed a significant relationship only with lymphocyte count. Thymic FDG uptake in pediatric patients after completion of chemotherapy should not be mistaken as recurrent or metastatic thymic malignancy. Thymic FDG uptake appears to be associated with thymic function or hematopoietic function in bone marrow. Interpretation of FDG-PET must be made with this consideration in mind.




Similar content being viewed by others
References
Kawabe J, Okamura T, Shakudo M, et al. Physiological FDG uptake in the palatine tonsils. Ann Nucl Med 2001; 15:297–300.
Cook GJ, Fogelman I, Maisey MN. Normal physiological and benign pathological variants of 18-fluoro-2-deoxyglucose positron-emission tomography scanning: potential for error in interpretation. Semin Nucl Med 1996; 26:308–314.
Engel H, Steinert H, Buck A, et al. Whole-body PET: physiological and artifactual fluorodeoxyglucose accumulations. J Nucl Med 1996; 37:441–446.
Patel PM, Alibazoglu H, Ali A, et al. Normal thymic uptake of FDG on PET imaging. Clin Nucl Med 1996; 21:772–775.
Brink I, Reinhardt MJ, Hoegerle S, et al. Increased metabolic activity in the thymus gland studied with18F-FDG PET: age dependency and frequency after chemotherapy. J Nucl Med. 2001; 42:591–595.
Weinblatt ME, Zanzi I, Belakhlef A, et al. False-positive FDG-PET imaging of the thymus of a child with Hodgkin’s disease. J Nucl Med 1997; 38:888–890.
Yoon SN, Park CH, Kim MK, et al. False-positive F-18 FDG gamma camera positron emission tomographic imaging resulting from inflammation of an anterior mediastinal mass in a patient with non-Hodgkin’s lymphoma. Clin Nucl Med 2001; 26:461–462.
Montravers F, McNamara D, Landman-Parker J, et al. [18F]FDG in childhood lymphoma: clinical utility and impact on management. Eur J Nucl Med Mol Imaging 2002; 29:1155–1165.
Shin MS, Ho KJ. Diffuse thymic hyperplasia following chemotherapy for nodular sclerosing Hodgkin’s disease. An immunologic rebound phenomenon? Cancer 1983; 51:30–33.
Choyke PL, Zeman RK, Gootenberg JE, et al. Thymic atrophy and regrowth in response to chemotherapy: CT evaluation. AJR 1987; 149:269–272.
Kissin CM, Husband JE, Nicholas D, et al. Benign thymic enlargement in adults after chemotherapy: CT demonstration. Radiology 1987; 163:67–70.
Bell BA, Esseltine DW, Azouz EM. Rebound thymic hyperplasia in a child with cancer. Med Pediatr Oncol 1984; 12:144–147.
Hamacher K, Coenen HH, Stoeclin G. Efficient stereospecific synthesis of no-carrier-added 2-[18F]-fluoro-2-deoxy-d-glucose using aminopolyether supported nucleophilic substitution. J Nucl Med 1986; 27:235–238.
Inoue T, Oriuchi N, Kunio M, et al. Accuracy of standardized uptake value(SUV) measured by simultaneous emission and transmission scanning in PET oncology. Nucl Med Commun 1999; 20:849–857.
Zasadny KR, Wahl RL. Standardized uptake values of normal tissues at PET with 2-[fluorine-18]-fluoro-2-deoxy-d-glucose: variations with body weight and a method for correction. Radiology 1993; 189:847–850.
Langer KJ, Braun U, Kops ER, et al. The influence of plasma glucose levels on fluorine-18-fluorodeoxyglucose uptake in bronchial carcinoma. J Nucl Med 1993; 34:355–359.
Mackall CL, Fleisher TA, Brown MR, et al. Lymphocyte depletion during treatment with intensive chemotherapy for cancer. Blood 1994; 84:2221–2228.
Mackall CL, Fleisher TA, Brown MR, et al. Distinctions between CD8+ and CD4+ T-cell regenerative pathways result in prolonged T-cell subset imbalance after intensive chemotherapy. Blood 1997; 89:3700–3707.
Mackall CL, Fleisher TA, Brown MR, et al. Age, thymopoiesis, and CD4+ T-lymphocyte regeneration after intensive chemotherapy. N Engl J Med 1995; 332:143–149.
Kubota R, Yamada S, Kubota K, et al. Intratumoral distribution of fluorine-18-dluorodeoxyglucose in vivo: high accumulation in macrophages and granulation tissue studied by microautoradiology. J Nucl Med 1992; 33:1972–1980.
Abbas AK, Lichtman AH. Cellular and molecular immunology, 5th edn. Philadelphia: Saunders, 2003.
Donahue DM, Leonard JC, Basmadjian GP, et al. Thymic gallium-67 localization in pediatric patients on chemotherapy: concise communication. J Nucl Med 1981; 22:1043–1048.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kawano, T., Suzuki, A., Ishida, A. et al. The clinical relevance of thymic fluorodeoxyglucose uptake in pediatric patients after chemotherapy. Eur J Nucl Med Mol Imaging 31, 831–836 (2004). https://doi.org/10.1007/s00259-004-1466-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-004-1466-4