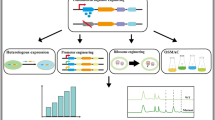
Abstract
Histone acetylation modifications in filamentous fungi play a crucial role in epigenetic gene regulation and are closely linked to the transcription of secondary metabolite (SM) biosynthetic gene clusters (BGCs). Histone deacetylases (HDACs) play a pivotal role in determining the extent of histone acetylation modifications and act as triggers for the expression activity of target BGCs. The genus Chaetomium is widely recognized as a rich source of novel and bioactive SMs. Deletion of a class I HDAC gene of Chaetomium olivaceum SD-80A, g7489, induces a substantial pleiotropic effect on the expression of SM BGCs. The C. olivaceum SD-80A ∆g7489 strain exhibited significant changes in morphology, sporulation ability, and secondary metabolic profile, resulting in the emergence of new compound peaks. Notably, three polyketides (A1–A3) and one asterriquinone (A4) were isolated from this mutant strain. Furthermore, our study explored the BGCs of A1–A4, confirming the function of two polyketide synthases (PKSs). Collectively, our findings highlight the promising potential of molecular epigenetic approaches for the elucidation of novel active compounds and their biosynthetic elements in Chaetomium species. This finding holds great significance for the exploration and utilization of Chaetomium resources.
Key points
• Deletion of a class I histone deacetylase activated secondary metabolite gene clusters.
• Three polyketides and one asterriquinone were isolated from HDAC deleted strain.
• Two different PKSs were reported in C. olivaceum SD-80A.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Filamentous fungi harbor numerous biosynthetic gene clusters (BGCs) responsible for the biosynthesis of secondary metabolites (SMs). The diversity within these BGCs offers significant potential for the synthesis of natural products. Several clinically employed antibacterials, antifungal agents, immunosuppressants, and antihypercholesterolemic drugs, including penicillin, anidulafungin, caspofungin, and lovastatin, originate from fungal natural products (Hoffmeister and Keller 2007; Newman and Cragg 2020). Chaetomium, a large genus within the fungal family Chaetomiaceae, is widely widespread in soil, air, and plants, and possesses both ecological and economic importance (Jiang et al. 2016). This fungus has been reported to synthesize more than 200 compounds, including depsidones, chaetoglobosins, terpenoids, and azaphilones (Zhang et al. 2012), many of which possess antimicrobial, antifungal, antitumor, and cytotoxic properties, among others (Zhang et al. 2012).
However, a significant proportion of these BGCs either remains unexpressed or exhibit very low expression under unnatural conditions. Therefore, activating the expression of these BGCs and acquiring the active substances are of great practical value (Rutledge and Challis 2015). Epigenetic modification of BGCs plays a crucial role in the biosynthesis of SMs. This process involves crucial mechanisms like DNA methylation and histone posttranslational modifications. Histones, which are the scaffolding proteins of eukaryotic chromosomes, directly influence the transcriptional activity of chromosomal DNA (Poças-Fonseca et al. 2020). Histones can be modified through multiple molecular mechanisms, including acetylation, methylation, phosphorylation, ubiquitination, adenylation, and ADP ribosylation, which collectively impact the transcriptional state of BGCs responsible for SM synthesis (Lai et al. 2022). Among these, histone acetylation and deacetylation are closely linked to the activation and silencing of gene transcription.
In Aspergillus nidulans, the knockout of the histone deacetylase (HDAC) gene hdaA successfully activated two secondary metabolic BGCs (Shwab et al. 2007). Additionally, inhibiting HDAC with hydroxamic acid has been reported to elevate the acetylation level of fungal SM genes, leading to the production of novel compounds (Asai et al. 2013). The knockout of the HDAC gene hdaA can activate up to 75% of SM synthetic genes in Calcarisporium arbuscula, resulting in the production of new structural compounds (Mao et al. 2015). Moreover, the elimination of hdaA from Penicillium chrysogenum enhances the activity of sorbicillinoids and meleagin/roquefortine BGCs, while downregulating the expression of naphtha-γ-pyrone and chrysogine synthetic genes (Ding et al. 2020; Guzman-Chavez et al. 2018). Deletion of a putative HDAC gene, hid1, in Pestalotiopsis microspore resulted in a twofold increase in the production of pestalotiollide B. (Niu et al. 2015). For the histone acetylation modification research on Chaetomium, chemical epigenetic modification using the NAD+-dependent HDAC inhibitor nicotinamide has been shown to stimulate the production of polyketides in Chaetomium cancroideum and Chaetomium mollipilium (Asai et al. 2012, 2016). Overexpression of histone acetyltransferases (HATs) activates dimeric bis-spiro-azaphilone BGC in Chaetomium globosum CBS148.51, and the deletion of HAT CgSptJ in this species results in the production of mollipilin A and B (Nakazawa et al. 2013; Wang et al. 2017). However, due to the inherently high non-homologous random recombination activity of Chaetomium, many Chaetomium strains are not suitable for molecular genetic manipulations, particularly gene knockout experiments (Wang et al. 2017). Moreover, the activation of silent BGCs in Chaetomium through HDAC knockout has not been reported, which severely limits the exploitation of compound and gene resources in Chaetomium.
Chaetomium olivaceum SD-80A was selected for further investigation due to its potent anti-methicillin-resistant Staphylococcus aureus (MRSA) activity, exhibiting a minimum inhibitory concentration (MIC) of 0.78 μg/mL. In a previous study, we isolated four compounds from the fermentation of C. olivaceum SD-80A, two of which demonstrated moderate bioactivity against S. aureus (SA) and MRSA (Wang et al. 2020). However, the number of compounds discovered from the strain is much lower than the number of BGCs predicted in silico. Based on these previous findings, our study sought to explore the use of genetic manipulation centered on HDAC genes as an effective strategy to activate fungal BGCs. Here, we performed knockout of the class I HDAC gene, g7489, in C. olivaceum SD-80A and explored the influence of its inactivation on secondary metabolism. Our findings revealed that the ∆g7489 strain exhibited significant alterations in morphology, sporulation ability, and the secondary metabolic profile, accompanied by the appearance of novel compound peaks. The differentially expressed compounds were identified as three polyketides (A1–A3) and one asterriquinone (A4). Heterologous expression in Aspergillus oryzae was then employed, which led to the identification of two polyketide synthases (PKSs) and an orsellinic acid (OA) derivative. Furthermore, we investigated the effect of HDAC on the SM biosynthetic core genes of C. olivaceum SD-80A. Therefore, this study not only sheds light on the regulatory role of HDAC in fungal secondary metabolism but also provides an effective strategy for the continued exploration and exploitation of Chaetomium-derived resources.
Materials and methods
Fungal material and growth media
The fungal strain C. olivaceum SD-80A was isolated from nilgai (Boselaphus tragocamelus) feces collected in New Delhi, India, and deposited at the Biology Institute, Shandong Academy of Sciences, Jinan, China and the China General Microbiological Culture Collection Center (CGMCC) (accession no. 40420). Daily culture was conducted using PDA and PDB medium.
Bioinformatics analysis
The genomic sequencing of C. olivaceum SD-80A was carried out on a PacBio Sequel instrument and subsequently assembled de novo with the Hierarchical Genome Assembly Process 3 (HGAP3) (Chin et al. 2013). The data was deposited in GenBank with accession no. PRJNA1065967. AntiSMASH and 2ndFind were used to predict the BGCs (Blin et al. 2023). Gene function prediction was conducted using the NCBI database. AUGUSTUS was used for gene prediction. Pfam was used for domain prediction (Bachmann and Ravel 2009). The Enzyme Function Initiative-Enzyme Similarity Tool (EFI-EST) was employed for constructing sequence similarity networks (SSNs) of proteins (Gerlt et al. 2015). Sequence alignment and phylogenetic analyses were performed with the MEGA 7.0 software by maximum likelihood method. The resulting phylogenetic tree was visualized using iTOL (Letunic and Bork 2021).
Generation of fungal strains
∆g7489 of C. olivaceum SD-80A was constructed as Szewczyk et al. described based on homologous recombination (Szewczyk et al. 2006). The flanking regions of the target gene were amplified from C. olivaceum SD-80A genome (Aidlab Biotechnologies Co. Ltd) and fused with the selection marker hygB.
C. olivaceum SD-80A was inoculated on a sporulation medium and cultured at 28 °C for 14 days. The spores were suspended in 100 mL PDB medium and shaken overnight at 28 °C. Transformations were carried out as described by Nakazawa et al. with minor modification (Nakazawa et al. 2013). Briefly, the mycelium was collected by filtration, washed three times with 15 mL of osmotic medium (OM), suspended in 15 mL OM containing 30 mg lysing enzymes (Sigma) and 20 mg Yatalase (Takara), and incubated at 28 °C for 4 h. The resulting protoplasts were filtered, added with 20 mL of protoplast trapping buffer, collected by centrifuging, resuspended in 300 μL STC buffer, and divided into tubes with 100 μL each. Five micrograms of DNA fragments was added to the protoplasts, incubated on ice for 40 min, added with 600 µL of PEG solution and incubated at room temperature for another 20 min. The mixture was plated on MYG–sorbitol agar medium with suitable antibiotics (200 μg/mL hygromycin B or G418). The genotype of the deletion mutant was confirmed via diagnostic PCR. For the complementation of g7489 (∆g7489-C), the ∆g7489 strain was co-transformed with a fragment containing the g7489 encoding sequence including the PtrpC promoter and a fragment comprising a geneticin selection marker G418, respectively. The primers are summarized in Table S1.
SM analysis of C. olivaceum SD-80A
The wild-type (WT) strain and ∆g7489 mutant were grown on PDA medium for 7 days. The mycelium from a Petri dish was transferred into three flasks, each containing 100 mL of PDB medium, for a 5-day seed culture period. Subsequently, the seed culture was inoculated into 30 flasks (60 mL of water and 40 g of rice per flask) for 30 days at 28 °C. The fermentation was extracted using a 1:1 volume ratio of ethyl acetate (EtOAc), and a residue was obtained after rotary evaporation at 38 °C. The solids obtained were dissolved in chromatographic grade methanol and analyzed by high-performance liquid chromatography (HPLC). The extracts were purified using silica gel column chromatography with eluents of petroleum ether: EtOAc/MeOH (methanol) (100:0:0, 80:20:0, 60:40:0, 40:60:0, 0:100:0, 0:90:10, 0:50:50, and 0:0:100, v/v) to yield eight fractions (Fr.1–8). Fr. 3 was subjected to fractionation on a Sephadex LH-20 column using an isocratic elution of dichloromethane (DCM)/MeOH (1:1) and produced five subfractions (3.1–3.5). Subfraction 3.3 underwent further fractionation by HPLC (Agilent C18 5 μm 10 × 250 mm, 2 mL/min, isocratic elution 53% MeOH/H2O) to collect compound 1 (A1). Fr. 4 was eluted under the same conditions as Fr. 3 and yielded six subfractions (4.1–4.6). Subfraction 4.5 was further fractionated into subfractions by HPLC to collect compounds 2 (A2), 3 (A3), and 4 (A4). The nuclear magnetic resonance (NMR) spectra of the compounds were recorded on a Bruker Biospin Avance 400 spectrometer. High-resolution-electrospray ionization-mass spectrometry (HR-ESI–MS) or ESI–MS data were recorded on an Agilent 6230 mass spectrometer (Agilent, USA).
Real-time PCR analysis
The fungi grown under the above-described fermentation conditions were harvested. Total RNA was extracted using Trizol. cDNA was synthesized with the HiScript III RT SuperMix for qPCR (+ gDNA wiper) (Vazyme, China). Real-time PCR was conducted on a Pangaea 6 Real-Time System (Apexbio, China). Three replicates of each cDNA sample were performed, and the average threshold cycle was calculated. Relative expression levels were determined via the 2−∆∆Ct method. Actin was used as the reference gene. Statistical analyses were performed using Microsoft Excel.
Heterologous expression in A. oryzae and metabolite detection
Genome DNA of C. olivaceum SD-80A was used to amplify PKS genes g657, g4635 and cytochrome P450 gene g656. The PCR products were inserted into linearized pUARA2 and pAdeA2 (treated by KpnI) using the ClonExpress Ultra One Step Cloning Kit (Vazyme, China) to generate the expression plasmids pUARA-N2.2, pUARA-N15.1, and pAdeA-P450. A. oryzae NSAR1 (niaD−, sC−, ∆argB, adeA−) was used as expression host. The transformations of A. oryzae were carried out as Tagami et al. described (Tagami et al. 2013).
Transformants were cultured in 10 mL MPY medium for 3 days at 30 °C, extracted three times with EtOAc, and the residue was obtained after rotary evaporation at 38 °C and 50 hPa. The residue was dissolved in chromatographic methanol and analyzed by HPLC (YMC-Pack ODS-A column, 10 × 250 mm, 5 μm). The HPLC analyses were conducted under the following conditions: gradient elution with 0.1% trifluoroacetic acid in H2O/MeCN (methyl cyanide) 95:5 to 100% MeCN in 30 min, MeCN 100% for 12 min, and a flow rate of 1 mL/min (Lackner et al. 2013).
Results
Gene sequencing and biological information mining
The genome of C. olivaceum SD-80A comprises 117 contigs, with a total length of 36,041,079 base pairs (bp) and a G + C content of 55.60%. Notably, the C. olivaceum genome harbors 46 BGCs responsible for the biosynthesis of SMs (Table S2). Among these clusters, we identified 14 type I PKS (T1PKS) clusters, 8 non-ribosomal peptide synthetase (NRPS) clusters, and 5 terpene clusters, along with other type clusters.
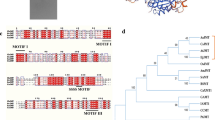
The HDAC protein (g7489) was identified through a BLAST search conducted in C. olivaceum SD-80A, using the sequence of the HDAC protein (XP_006695713.1) reported in Thermochaetoides thermophila DSM 1495. The g7489 protein, encoded by a 1491 bp genomic sequence containing no intron, comprises 496 amino acids and features a conserved domain of the arginase_HDAC superfamily. The nucleotide sequence was deposited in GenBank with accession number PP498810. Significantly, this protein is homologous to the class I HDAC Hos2, sharing a high degree of similarity with orthologs from T. thermophila (82.73% identity) and Neurospora crassa (75.91% identity), respectively. A phylogenetic analysis further revealed that g7489 and its orthologs across diverse fungal species exhibit evolutionary conservation (Fig. S1).
Generation of the HDAC gene knockout mutant strain and its complementation in C. olivaceum SD-80A
Deletion mutants were generated by replacing the HDAC gene g7489 with the hygromycin-resistance gene, serving as a selectable marker, in the WT strain of C. olivaceum SD-80A (Fig. S2a). Deletion mutants were identified through PCR analysis using five primer pairs 5-F-out/3-R-out, 5-F-out/Y-HygR-R, Y-HygR-DF/3-R-out, PJ007/PJ008, and F-in/R-in (Fig. S2b, Table S1). The ∆g7489 mutants were further verified through PCR product sequencing. To complement the strain, transformants (∆g7489-C1-C6) were created by introducing the target gene with the PtrpC promoter into the genome of the ∆g7489 mutant strain. Verification was conducted by screening for geneticin resistance and PCR targeting the g7489 gene using the PJ007/g7489-R-out primer pair (Fig. S2c, Table S1).
Effect of g7489 deletion on the phenotype, growth, and SMs of C. olivaceum SD-80A
To assess the impact of g7489 deletion on the phenotype and growth of C. olivaceum SD-80A, we cultivated ∆g7489, WT, and ∆g7489-C strains on both PDA and sporulation medium. As illustrated in Fig. 1a, the mycelium of ∆g7489 exhibited increased thickness and fluffiness, with clearly visible white mycelium masses. In contrast, little difference in mycelial growth and morphology was observed between WT and ∆g7489-C. WT and ∆g7489-C started to produce ascomata and spores in approximately 2 weeks, while ∆g7489 was incapable of spore production (Fig. 1b and Fig. S3). The number of spores per Petri dish (90 × 15 mm) was approximately 1 × 1010 for WT and ∆g7489-C. These findings demonstrated that g7489 plays a crucial role in spore formation, but it does not seem to be essential for vegetative growth. HPLC profiling of SMs from ∆g7489 revealed significant alterations compared to the WT strain (Fig. 2). Notably, several novel peaks were observed in the HPLC profiling of mutant extracts, indicating that g7489 significantly influenced the SM profile of C. olivaceum SD-80A.
Scale-up fermentation was conducted to characterize the differentially produced compounds between ∆g7489 and WT. Compounds 1–4 (A1–A4) were determined as OA (A1) (Kloss and Clayton 1965), globosumone C (A2) (Bashyal et al. 2005), orsellide A (A3) (Schlörke and Zeeck 2006), and cochliodinol (A4) (Kingsland and Barrow 2009), by ESI–MS and NMR spectra (Fig. 2, Fig. S4-S15). A1 is an aromatic polyketide. A2 and A3 are ester derivatives of A1. A4 is an asterriquinone. Other peaks in the HPLC profiles were not identified due to their low concentration.
Putative BGC of A4
A4 is biosynthesized by an indole-type cluster. Given that there was only one indole-type cluster (Table S1, cluster 3.2) in C. olivaceum SD-80A, we compared the cluster with the known cochliodinol BGC in C. globosum and found that cluster 3.2 exhibited high similarity to that in C. globosum (Fig. S16, Table S3) (Nakazawa et al. 2013). Most of the genes in the cochliodinol BGC have its homologous genes in cluster 3.2. The proposed core gene for cochliodinol biosynthesis, which encodes the indole prenyltransferase (encoded by g1124), exhibited 89.61% identity with CHGG_03684 from C. globosum. According to antiSMASH, 60% of the genes in cluster 3.2 exhibited similarity to the terrequinone A BGC. As expected, the mRNA expression level of g1124 was upregulated 7.08-fold in ∆g7489 compared to that in WT by real-time PCR (Fig. 3, Indol3.2).
Putative BGCs of A1–A3
A1 is formed through the condensation of acetyl-CoA and malonyl-CoA by PKS (Tao and Abe 2021). In fungi and bacteria, the biosynthesis of A1 is mediated by non-reducing PKS (NRPKS) (Jørgensen et al. 2014; Sanchez et al. 2010). A2 and A3 are ester derivatives of A1. The biosynthesis of OA derivatives depends on key enzymes such as PKS for generating the polyketide moiety and esterase or cytochrome P450 for forming the ester bond. Previous studies have indeed reported that some PKSs also possess esterification functionality (Kealey et al. 2021). C. olivaceum SD-80A harbors 19 T1PKSs, encompassing 13 highly reducing PKSs (HRPKSs) and 6 NRPKSs. Among these PKSs, HRPKSs of clusters 1.1, 2.2, 4.2, 6.1, 6.2, and 14.1 (H1.1, H2.2–2, H4.2, H6.1, H6.2, and H14.1) and NRPKSs of clusters 2.2, 3.1, 7.4, and 15.1 (N2.2, N3.1, N7.4, and N15.1) were upregulated (Fig. 3). The domain organization of the PKSs is shown in Fig. S17 and Table S4. N2.2 (encoded by g657) and N15.1 (encoded by g4635) contain a starter-unit acetyltransferase (SAT), a ketosynthase (KS), an acyltransferase (AT), a product template (PT), one or two acyl carrier protein (ACP), and a thioesterase (TE) domain (Fig. S17), and their domain organization was the same with orsellinic acid synthases (OASs), which can produce OA, such as ArmB and OrsA (Lackner et al. 2013; Sanchez et al. 2010). Therefore, the two NRPKSs were regarded as potential candidates responsible for the biosynthesis of A1–A3. The mRNA expression levels of g657 and g4635 were upregulated by 1.73- and 1.63-fold, respectively, in ∆g7489 compared to that in WT, which was consistent with the upregulated compound products. The gene organization of cluster 2.2 and 15.1 is shown in Fig. S18 and Table S5-S6. Cluster 2.2 contains a cytochrome P450, while there is no related post-modification enzyme in cluster 15.1.
Sequence analysis revealed that the N2.2 and N15.1 coding genes (g657 and g4635) had open reading frames of 6601 bp and 6809 bp, encoding polypeptides of 2044 and 2147 amino acids with estimated molecular weights of 222.05 kDa and 235.24 kDa, respectively (deposited as GenBank accession numbers PP068241 and PP068240, Results S1–S4). For better visualization and classification, SSNs were constructed for approximately 1000 NRPKS sequences using N2.2 and N15.1 as queries (Fig. S19 and S20). NRPKSs from Fusarium and Aspergillus were the most common, with sequences predominantly grouped by genus. Despite N2.2 and N15.1 being from Chaetomium, only a limited number of sequences were identified from this genus, suggesting a need for further exploration of PKSs in Chaetomium. N2.2 can be clustered with the reported OASs ArmB and OpS1 (Feng et al. 2015; Lackner et al. 2013). Additionally, NRPKS TerA can be found in N2.2 and N15.1 SSNs (Zaehle et al. 2014). Phylogenetic analysis, based on complete amino acid sequences and KS domain sequences, revealed that N2.2 is closely related to enzymes involved in OA biosynthesis, particularly PKS14 and OpS1 (Fig. S21-S23, Table S7) (Feng et al. 2015; Jørgensen et al. 2014), while N15.1 is closely related to TerA (Fig. S21 and S22) (Zaehle et al. 2014). A comparison of the amino acid sequences showed that the overall sequence identity of N2.2 and PKS14 was 51.18% (N2.2 and Ops1, 35.15%; N15.1 and TerA, 41.18%), and the KS domain sequence identity of N2.2 and PKS14 was 74.65% (N2.2 and Ops1, 57.10%; N15.1 and TerA, 64.84%), whereas the TE domain sequence identity of N2.2 and PKS14 was 51.85% (N2.2 and Ops1, 50.00%; N15.1 and TerA, 38.24%).
Heterologous expression of the complete gene sequences of N2.2 and N15.1 in A. oryzae (pUARA-N2.2 and pUARA-N15.1) was conducted, and HPLC analysis of fermentation extracts revealed the emergence of novel peaks (Fig. 4). The new peak in pUARA-N2.2 was determined to be OA (m/z 167.0 [M – H]−) by ESI–MS. One of the new peaks in pUARA-N15.1 was also OA. The other new peak (compound B1) was determined to be 3,6,8-trihydroxy-3-methyl-3,4-dihydroisocoumarin by HR-ESI–MS and NMR (Ariantari et al. 2020; Kameda et al. 1973) (Fig. S24-S26). Consequently, both N2.2 and N15.1 possess the capacity to catalyze the synthesis of OA, with N15.1 being a multifunctional enzyme. Notably, the evolutionary distance between the two proteins is relatively large. Cytochrome P450 in cluster 2.2 was heterologously expressed in pUARA-N2.2 transformant, and no OA derivatives were detected (Fig. 4), which may be due to the lack of deoxyhexose in A. oryzae.
Discussion
The genus Chaetomium encompasses numerous species and is widely distributed, with its natural products exhibiting rich and diverse structures. Many SMs with biological activity have been discovered in Chaetomium, such as chaetoglobosins, depsidones, epipolythiodioxopiperazines, and azaphilones (Qi et al. 2020; Zhang et al. 2012; Zhao et al. 2022, 2021). However, despite the large number of BGCs predicted by genomic information in Chaetomium, only a small proportion of the compounds derived from these fungi and their specific BGCs have been identified. Therefore, thoroughly exploring the genetic and compound resources of Chaetomium is of great significance. As key molecules in eukaryotic gene expression, HDACs play a crucial role in regulating many fungal proteins and can influence SM BGCs (Pidroni et al. 2018). Using HDAC as a target to activate silent BGCs represents a crucial molecular epigenetic approach for mining active compounds in Chaetomium.
In our previous study, a C. olivaceum SD-80A strain with anti-MRSA activity (MIC: 0.78 μg/mL) was obtained, and four polyketides were identified in its WT strain, including three new compounds (Wang et al. 2020). However, bioinformatics analysis of C. olivaceum SD-80A revealed 46 BGCs, far exceeding the number of discovered compounds. By knocking out the class I HDAC gene g7489, four compounds (A1–A4) were obtained. A4, an asterriquinone, reached a yield of 385 mg/kg in the ∆g7489 strain, 12.03 times that of the WT. A4 was biosynthesized by an indole-type cluster, and by comparing with reported BGCs (Nakazawa et al. 2013), it was hypothesized that A4 is synthesized by the upregulated cluster 3.2 in ∆g7489. A4 exhibited antimicrobial activities against Candida albicans ATCC 10213, S. aureus ATCC 29213, and Enterococcus faecalis, as well as cytotoxic activity against the human cell lines KB (uterine cervical carcinoma), MDA-MB-435 (melanoma), and MRC5 (normal human lung fibroblasts) (Casella et al. 2013; Kingsland and Barrow 2009), suggesting that this compound could have clinical applications.
Moreover, a series of polyketides were obtained by knocking out the HDAC gene of C. olivaceum SD-80A. A2 exhibited moderate acetylcholinesterase inhibitory activity with a half maximal inhibitory concentration (IC50) value of 7.34 μΜ (Xu et al. 2018). A1 is an essential intermediate in pharmaceutical production. Over 200 OA derivatives with biological activities have been found in plants, lichens, fungi, and bacteria (Chen et al. 2020). For example, mycophenolic acid, derived from the Penicillium brevicompactum, is widely used as a first-line immunosuppressive drug (Zhang et al. 2019). Moreover, ascofuranone, which is derived from Acremonium egyptiacum, is a promising drug candidate against African trypanosomiasis (Araki et al. 2019). To investigate the biosynthesis of A1–A3, all PKSs in C. olivaceum SD-80A were characterized through bioinformatics analysis and RT-PCR experiments. OA is catalyzed by repetitive T1PKS in fungi and bacteria (Ahlert et al. 2002; Han et al. 2023; Sanchez et al. 2010; Weitnauer et al. 2001), whereas in plants, it is produced by T3PKS (Taura et al. 2016). Two PKSs, N2.2 and N15.1, capable of synthesizing OA were identified in C. olivaceum SD-80A, exhibiting a significant evolutionary distance and clustering with distinct PKSs in the phylogenetic tree (Fig. S21-S23). N15.1 clustered with TerA in the phylogenetic tree, sharing the same domain organization and a similarity sequence identity of 41.18%. TerA, found in Aspergillus terreus, can produce 4-hydroxy-6-methylpyranone (4-HMP), OA, and 2,3-dehydro-6-hydroxymellein (2,3-dehydro-6-HM), condensing acetyl-CoA with two, three, or four malonyl-CoA units (Zaehle et al. 2014). In contrast, N15.1 produced OA and 3,6,8-trihydroxy-3-methyl-3,4-dihydroisocoumarin (B1), another OA derivative. However, B1 was not found in WT or ∆g7489 of C. olivaceum SD-80A, which is probably due to its low yield or unknown post modification. N2.2 clustered with PKS14 from Fusarium graminearum and OpS1 from Beauveria bassiana in the phylogenetic tree and with OpS1 and ArmB from Armillaria mellea in SSN. The gene organization of cluster 2.2, cluster 15.1, and those of TerA, PKS14, and OpS1 showed significant differences, which may indicate that these BGCs branched out in the evolution of different species, resulting in different end products.
No OA esters were detected in A. oryzae despite the presence of N2.2 or N15.1. This absence could be attributed to the inability of both N2.2 and N15.1 to catalyze the formation of ester bonds in A2 and A3. Additionally, the lack of deoxyhexose in A. oryzae and the strong substrate specificity of these enzymes might also contribute to this observation. Despite the co-expression of cytochrome P450 in cluster 2.2 with N2.2 in A. oryzae, no novel esterification products were generated. Schlörke and Zeeck reported that the sugar part of A3 was derived from glucose (Schlörke and Zeeck 2006). Since no OA esters were obtained in A. oryzae, it was hypothesized that C. olivaceum SD-80A possesses specific enzymes capable of synthesizing deoxyhexose, which were apparently absent in A. oryzae. Deoxyhexoses serve as typical building blocks of bacterial SMs and play a crucial role in regulating their biological activities, particularly in the case of macrolides (Fernández et al. 1998; Schlörke and Zeeck 2006). These sugar moieties often contribute to the unique pharmacological properties and bioactivities of these compounds. While fungi generally do not produce SMs containing deoxyhexose, only a few fungal metabolites have been reported, such as sordarin and its derivatives (Weber et al. 2005). Bacteria typically employ enzymes like glucose-1-phosphate thymidylyltransferase (e.g., RmlA) and dTDP-d-glucose-4,6-dehydratase (e.g., RmlB) to catalyze the conversion of glucose-1-phosphate into dTDP-4-oxo-6-deoxy-d-glucose. Subsequently, enzymes such as dTDP-4-oxo-6-deoxy-d-glucose-3,4-oxoisomerase (e.g., QdtA) are utilized to further transform this intermediate into dTDP-3-oxo-6-deoxy-d-glucose, the possible sugar moiety of A3 (Pföstl et al. 2008). Surprisingly, the orthologous protein of QdtA was not found in C. olivaceum SD-80A or A. oryzae, which means that it can produce deoxyhexose in C. olivaceum SD-80A by a catalytic mechanism that has not been described in fungi. The homologous BGCs of clusters 2.2 and 15.1 activated via the deletion of class I HDAC g7489 have not yet been found in other fungi. Cluster 2.2 contains up to 33 genes, including one NRPKS and two HRPKSs, and most genes have no predicted functions. In addition, the low substrate specificity of N15.1 is also relatively unique and warrants further investigation. Due to the high non-homologous recombination rate of Chaetomium, we have not yet obtained knockout strains of N2.2 and N15.1. It has been reported that in order to enhance homologous recombination efficiency, it is essential to knock out genes involved in non-homologous random recombination activity, such as LigD or Ku70 (Nakazawa et al. 2013); thus, it is necessary to develop a gene-editing system suitable for Chaetomium. Meanwhile, the heterologous expression in A. oryzae to verify gene function is a relatively efficient method for Chaetomium. Therefore, a more efficient way to exploit Chaetomium resources is to use global regulators such as HDAC to activate silent BGCs and then use heterologous expression to investigate targeted gene functions, mine new compounds, and deduce their biosynthetic pathway.
Data availability
All data generated or analyzed during this study are included in this published article (and its supplementary information file).
References
Ahlert J, Shepard E, Lomovskaya N, Zazopoulos E, Staffa A, Bachmann BO, Huang K, Fonstein L, Czisny A, Whitwam RE, Farnet CM, Thorson JS (2002) The calicheamicin gene cluster and its iterative type I enediyne PKS. Science 297(5584):1173–1176. https://doi.org/10.1126/science.1072105
Araki Y, Awakawa T, Matsuzaki M, Cho R, Matsuda Y, Hoshino S, Shinohara Y, Yamamoto M, Kido Y, Inaoka DK, Nagamune K, Ito K, Abe I, Kita K (2019) Complete biosynthetic pathways of ascofuranone and ascochlorin in Acremonium egyptiacum. P Natl Acad Sci USA 116(17):8269–8274. https://doi.org/10.1073/pnas.1819254116
Ariantari NP, Ancheeva E, Frank M, Stuhldreier F, Meier D, Gröner Y, Reimche I, Teusch N, Wesselborg S, Müller WEG, Kalscheuer R, Liu Z, Proksch P (2020) Didymellanosine, a new decahydrofluorene analogue, and ascolactone C from Didymella sp. IEA-3B.1, an endophyte of Terminalia catappa. RSC Adv 10(12):7232–7240. https://doi.org/10.1039/C9RA10685E
Asai T, Morita S, Shirata N, Taniguchi T, Monde K, Sakurai H, Ozeki T, Oshima Y (2012) Structural diversity of new C13-polyketides produced by Chaetomium mollipilium cultivated in the presence of a NAD+-dependent histone deacetylase inhibitor. Org Lett 14(21):5456–5459. https://doi.org/10.1021/ol302539s
Asai T, Yamamoto T, Shirata N, Taniguchi T, Monde K, Fujii I, Gomi K, Oshima Y (2013) Structurally diverse chaetophenol productions induced by chemically mediated epigenetic manipulation of fungal gene expression. Org Lett 15(13):3346–3349. https://doi.org/10.1021/ol401386w
Asai T, Morita S, Taniguchi T, Monde K, Oshima Y (2016) Epigenetic stimulation of polyketide production in Chaetomium cancroideum by an NAD+-dependent HDAC inhibitor. Org Biomol Chem 14(2):646–651. https://doi.org/10.1039/C5OB01595B
Bachmann BO, Ravel J (2009) Chapter 8. Methods for in silico prediction of microbial polyketide and nonribosomal peptide biosynthetic pathways from DNA sequence data. Methods Enzymol 458:181–217. https://doi.org/10.1016/S0076-6879(09)04808-3
Bashyal BP, Wijeratne EMK, Faeth SH, Gunatilaka AAL (2005) Globosumones A−C, cytotoxic orsellinic acid esters from the sonoran desert endophytic fungus Chaetomium globosum. J Nat Prod 68(5):724–728. https://doi.org/10.1021/np058014b
Blin K, Shaw S, Augustijn HE, Reitz ZL, Biermann F, Alanjary M, Fetter A, Terlouw BR, Metcalf WW, Helfrich EJN, van Wezel GP, Medema MH, Weber T (2023) antiSMASH 7.0: new and improved predictions for detection, regulation, chemical structures and visualisation. Nucleic Acids Res 51(W1):W46–W50. https://doi.org/10.1093/nar/gkad344
Casella TM, Eparvier V, Mandavid H, Bendelac A, Odonne G, Dayan L, Duplais C, Espindola LS, Stien D (2013) Antimicrobial and cytotoxic secondary metabolites from tropical leaf endophytes: isolation of antibacterial agent pyrrocidine C from Lewia infectoria SNB-GTC2402. Phytochemistry 96:370–377. https://doi.org/10.1016/j.phytochem.2013.10.004
Chen GD, Hu D, Huang MJ, Tang J, Wang XX, Zou J, Xie J, Zhang WG, Guo LD, Yao XS, Abe I, Gao H (2020) Sporormielones A-E, bioactive novel C–C coupled orsellinic acid derivative dimers, and their biosynthetic origin. Chem Commun 56(33):4607–4610. https://doi.org/10.1039/D0CC00855A
Chin CS, Alexander DH, Marks P, Klammer AA, Drake J, Heiner C, Clum A, Copeland A, Huddleston J, Eichler EE (2013) Nonhybrid, finished microbial genome assemblies from long-read SMRT sequencing data. Nat Methods 10(6):563–569. https://doi.org/10.1038/nmeth.2474
Ding Z, Zhou H, Wang X, Huang H, Wang H, Zhang R, Wang Z, Han J (2020) Deletion of the histone deacetylase HdaA in endophytic fungus Penicillium chrysogenum Fes1701 induces the complex response of multiple bioactive secondary metabolite production and relevant gene cluster expression. Molecules (basel, Switzerland) 25(16):3657. https://doi.org/10.3390/molecules25163657
Feng P, Shang Y, Cen K, Wang C (2015) Fungal biosynthesis of the bibenzoquinone oosporein to evade insect immunity. P Natl Acad Sci USA 112(36):11365–11370. https://doi.org/10.1073/pnas.1503200112
Fernández E, Weissbach U, Sánchez Reillo C, Braña AF, Méndez C, Rohr J, Salas JA (1998) Identification of two genes from Streptomyces argillaceus encoding glycosyltransferases involved in transfer of a disaccharide during biosynthesis of the antitumor drug mithramycin. J Bacteriol 180(18):4929–4937. https://doi.org/10.1128/jb.180.18.4929-4937.1998
Gerlt JA, Bouvier JT, Davidson DB, Imker HJ, Sadkhin B, Slater DR, Whalen KL (2015) Enzyme Function Initiative-Enzyme Similarity Tool (EFI-EST): A web tool for generating protein sequence similarity networks. Biochim Biophys Acta 1854(8):1019–1037. https://doi.org/10.1016/j.bbapap.2015.04.015
Guzman-Chavez F, Salo O, Samol M, Ries M, Kuipers J, Bovenberg RAL, Vreeken RJ, Driessen AJM (2018) Deregulation of secondary metabolism in a histone deacetylase mutant of Penicillium chrysogenum. MicrobiologyOpen 7(5):e00598. https://doi.org/10.1002/mbo3.598
Han H, Yu C, Qi J, Wang P, Zhao P, Gong W, Xie C, Xia X, Liu C (2023) High-efficient production of mushroom polyketide compounds in a platform host Aspergillus oryzae. Microb Cell Fact 22(1):60. https://doi.org/10.1186/s12934-023-02071-9
Hoffmeister D, Keller NP (2007) Natural products of filamentous fungi: enzymes, genes, and their regulation. Nat Prod Rep 24(2):393–416. https://doi.org/10.1039/b603084j
Jiang T, Wang M, Li L, Si J, Song B, Zhou C, Yu M, Wang X, Zhang Y, Ding G, Zou Z (2016) Overexpression of the global regulator LaeA in Chaetomium globosum leads to the biosynthesis of chaetoglobosin Z. J Nat Prod 79(10):2487–2494. https://doi.org/10.1021/acs.jnatprod.6b00333
Jørgensen SH, Frandsen RJN, Nielsen KF, Lysøe E, Sondergaard TE, Wimmer R, Giese H, Sørensen JL (2014) Fusarium graminearum PKS14 is involved in orsellinic acid and orcinol synthesis. Fungal Genet Biol 70:24–31. https://doi.org/10.1016/j.fgb.2014.06.008
Kameda K, Aoki H, Tanaka H, Namiki M (1973) Studies on metabolites of Alternaria kikuchiana Tanaka, a phytopathogenic fungus of Japanese pear. Agric Biol Chem 37(9):2137–2146. https://doi.org/10.1080/00021369.1973.10860946
Kealey JT, Craig JP, Barr PJ (2021) Identification of a lichen depside polyketide synthase gene by heterologous expression in Saccharomyces cerevisiae. Metab Eng Commun 13:e00172. https://doi.org/10.1016/j.mec.2021.e00172
Kingsland S, Barrow R (2009) Identification of chaetoviridin E from a cultured microfungus, Chaetomium sp. and structural reassignment of chaetoviridins B and D. Aust J Chem 62:269–274. https://doi.org/10.1071/CH08259
Kloss RA, Clayton DA (1965) A synthesis of orsellinic acid. J Org Chem 30(10):3566–3567. https://doi.org/10.1021/jo01021a507
Lackner G, Bohnert M, Wick J, Hoffmeister D (2013) Assembly of melleolide antibiotics involves a polyketide synthase with cross-coupling activity. Chem Biol 20(9):1101–1106. https://doi.org/10.1016/j.chembiol.2013.07.009
Lai Y, Wang L, Zheng W, Wang S (2022) Regulatory roles of histone modifications in filamentous fungal pathogens. J Fungi 8(6):565. https://doi.org/10.3390/jof8060565
Letunic I, Bork P (2021) Interactive Tree Of Life (iTOL) v5: an online tool for phylogenetic tree display and annotation. Nucleic Acids Res 49(W1):W293–W296. https://doi.org/10.1093/nar/gkab301
Mao XM, Xu W, Li D, Yin WB, Chooi YH, Li YQ, Tang Y, Hu Y (2015) Epigenetic genome mining of an endophytic fungus leads to the pleiotropic biosynthesis of natural products. Angew Chem Int Ed 54(26):7592–7596. https://doi.org/10.1002/anie.201502452
Nakazawa T, Ki I, Sato M, Tsunematsu Y, Sugimoto S, Gotanda Y, Noguchi H, Hotta K, Watanabe K (2013) Targeted disruption of transcriptional regulators in Chaetomium globosum activates biosynthetic pathways and reveals transcriptional regulator-like behavior of aureonitol. J Am Chem Soc 135(36):13446–13455. https://doi.org/10.1021/ja405128k
Newman DJ, Cragg GM (2020) Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J Nat Prod 83(3):770–803. https://doi.org/10.1021/acs.jnatprod.9b01285
Niu X, Hao X, Hong Z, Chen L, Yu X, Zhu X (2015) A putative histone deacetylase modulates the biosynthesis of pestalotiollide B and conidiation in Pestalotiopsis microspora. J Microbiol Biotechnol 25(5):579–588. https://doi.org/10.4014/jmb.1409.09067
Pföstl A, Zayni S, Hofinger A, Kosma P, Schäffer C, Messner P (2008) Biosynthesis of dTDP-3-acetamido-3,6-dideoxy-alpha-D-glucose. Biochem J 410(1):187–194. https://doi.org/10.1042/bj20071044
Pidroni A, Faber B, Brosch G, Bauer I, Graessle S (2018) A class 1 histone deacetylase as major regulator of secondary metabolite production in Aspergillus nidulans. Front Microbiol 9:2212. https://doi.org/10.3389/fmicb.2018.02212
Poças-Fonseca MJ, Cabral CG, Manfrão-Netto JHC (2020) Epigenetic manipulation of filamentous fungi for biotechnological applications: a systematic review. Biotechnol Lett 42(6):885–904. https://doi.org/10.1007/s10529-020-02871-8
Qi J, Jiang L, Zhao P, Chen H, Jia X, Zhao L, Dai H, Hu J, Liu C, Shim SH, Xia X, Zhang L (2020) Chaetoglobosins and azaphilones from Chaetomium globosum associated with Apostichopus japonicus. Appl Microbiol Biotechnol 104(4):1545–1553. https://doi.org/10.1007/s00253-019-10308-0
Rutledge PJ, Challis GL (2015) Discovery of microbial natural products by activation of silent biosynthetic gene clusters. Nat Rev Microbiol 13:509–523. https://doi.org/10.1038/nrmicro3496
Sanchez JF, Chiang YM, Szewczyk E, Davidson AD, Ahuja M, Elizabeth Oakley C, Woo Bok J, Keller N, Oakley BR, Wang CC (2010) Molecular genetic analysis of the orsellinic acid/F9775 gene cluster of Aspergillus nidulans. Mol Biosyst 6(3):587–593. https://doi.org/10.1039/b904541d
Schlörke O, Zeeck A (2006) Orsellides A-E: an example for 6-deoxyhexose derivatives produced by fungi. Eur J Org Chem 4:1043–1049. https://doi.org/10.1002/ejoc.200500793
Shwab EK, Bok JW, Tribus M, Galehr J, Graessle S, Keller NP (2007) Histone deacetylase activity regulates chemical diversity in Aspergillus. Eukaryot Cell 6(9):1656–1664. https://doi.org/10.1128/EC.00186-07
Szewczyk E, Nayak T, Oakley CE, Edgerton H, Xiong Y, Taheri-Talesh N, Osmani SA, Oakley BR (2006) Fusion PCR and gene targeting in Aspergillus nidulans. Nat Protoc 1(6):3111–3120. https://doi.org/10.1038/nprot.2006.405
Tagami K, Liu C, Minami A, Noike M, Isaka T, Fueki S, Shichijo Y, Toshima H, Gomi K, Dairi T, Oikawa H (2013) Reconstitution of biosynthetic machinery for indole-diterpene paxilline in Aspergillus oryzae. J Am Chem Soc 135(4):1260–1263. https://doi.org/10.1021/ja3116636
Tao H, Abe I (2021) Enzymology and biosynthesis of the orsellinic acid derived medicinal meroterpenoids. Curr Opin Biotechnol 69:52–59. https://doi.org/10.1016/j.copbio.2020.11.016
Taura F, Iijima M, Yamanaka E, Takahashi H, Kenmoku H, Saeki H, Morimoto S, Asakawa Y, Kurosaki F, Morita H (2016) A novel class of plant type III polyketide synthase involved in orsellinic acid biosynthesis from Rhododendron dauricum. Front Plant Sci 7:1452. https://doi.org/10.3389/fpls.2016.01452
Wang MH, Jiang T, Ding G, Niu SB, Wang XW, Yu M, Gu YC, Zhang QB, Chen JH, Jia HM, Zou ZM (2017) Molecular epigenetic approach activates silent gene cluster producing dimeric bis-spiro-azaphilones in Chaetomium globosum CBS148.51. J Antibiot 70(6):801–804. https://doi.org/10.1038/ja.2017.4
Wang X, Zhao L, Liu C, Qi J, Zhao P, Liu Z, Li C, Hu Y, Yin X, Liu X, Liao Z, Zhang L, Xia X (2020) New tetramic acids comprising of decalin and pyridones from Chaetomium olivaceum SD-80A with antimicrobial activity. Front Microbiol 10:2958. https://doi.org/10.3389/fmicb.2019.02958
Weber RW, Meffert A, Anke H, Sterner O (2005) Production of sordarin and related metabolites by the coprophilous fungus Podospora pleiospora in submerged culture and in its natural substrate. Mycol Res 109(Pt 5):619–626. https://doi.org/10.1017/s0953756205002765
Weitnauer G, Mühlenweg A, Trefzer A, Hoffmeister D, Süssmuth R, Jung G, Welzel K, Vente A, Girreser U, Bechthold A (2001) Biosynthesis of the orthosomycin antibiotic avilamycin A: deductions from the molecular analysis of the avi biosynthetic gene cluster of Streptomyces viridochromogenes Tü57 and production of new antibiotics. Chem Biol 8:569–581. https://doi.org/10.1016/S1074-5521(01)00040-0
Xu QL, Xiao YS, Shen Y, Wu HM, Zhang X, Deng XZ, Wang TT, Li W, Tan RX, Jiao RH, Ge HM (2018) Novel chaetospirolactone and orsellide F from an endophytic fungus Chaetomium sp. J Asian Nat Prod Res 20(3):234–241. https://doi.org/10.1080/10286020.2017.1320548
Zaehle C, Gressler M, Shelest E, Geib E, Hertweck C, Brock M (2014) Terrein biosynthesis in Aspergillus terreus and its impact on phytotoxicity. Chem Biol 21(6):719–731. https://doi.org/10.1016/j.chembiol.2014.03.010
Zhang Q, Li HQ, Zong SC, Gao JM, Zhang AL (2012) Chemical and bioactive diversities of the genus Chaetomium secondary metabolites. Mini Rev Med Chem 12(2):127–148. https://doi.org/10.2174/138955712798995066
Zhang W, Du L, Qu Z, Zhang X, Li F, Li Z, Qi F, Wang X, Jiang Y, Men P, Sun J, Cao S, Geng C, Qi F, Wan X, Liu C, Li S (2019) Compartmentalized biosynthesis of mycophenolic acid. P Natl Acad Sci USA 116(27):13305–13310. https://doi.org/10.1073/pnas.1821932116
Zhao P, Yang M, Zhu G, Zhao B, Wang H, Liu H, Wang X, Qi J, Yin X, Yu L, Meng Y, Li Z, Zhang L, Xia X (2021) Mollicellins S-U, three new depsidones from Chaetomium brasiliense SD-596 with anti-MRSA activities. J Antibiot 74(5):317–323. https://doi.org/10.1038/s41429-020-00398-8
Zhao P, Liu H, Wu Q, Meng Q, Qu K, Yin X, Wang M, Zhao X, Qi J, Meng Y, Xia X, Zhang L (2022) Investigation of chetomin as a lead compound and its biosynthetic pathway. Appl Microbiol Biotechnol 106(8):3093–3102. https://doi.org/10.1007/s00253-022-11925-y
Acknowledgements
We thank Prof. Hideaki Oikawa (Hokkaido University) for providing A. oryzae NASR1 and the expression vectors pUARA2 and pAdeA2.
Funding
This work was supported by the Funding Key R&D Program of Shandong Province, China (2022SFGC0105); Natural Science Foundation of Shandong Province (No. ZR2022QC186, ZR2021QB173, ZR2021LSW022, ZR2023QB021); and Young Taishan Scholarship to Xuekui Xia (tsqn202103100), Jinan Talent Project for Universities (2021GXRC062, 202228088), Key Innovation Project and Science, Education and Industry Integration Innovation Pilot Project Qilu University of Technology (Shandong Academy of Sciences) (2022PX051, 2022PX027, 2022JBZ01-06, 2022GH024), and Research Project for Talents Qilu University of Technology (Shandong Academy of Sciences) (2023RCKY214).
Author information
Authors and Affiliations
Contributions
P.Z., X.X., and L.Z. designed the study and wrote the manuscript. P.Z., X.X., and L.Z. designed the study and wrote the manuscript. P.Z. conducted the gene knockout and complementation experiments. S.C., J.W., and H.L. isolated and identified the compounds. J.L. and Q.Z. carried out the heterologous expression experiments. Y.Z., C.L., Y.M., X.Y., M.W., and J.Q. conducted the bioinformatics analysis and critically revised the manuscript. All authors discussed the results. X.X. and L.Z. designed and supervised the project. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhao, P., Cao, S., Wang, J. et al. Activation of secondary metabolite gene clusters in Chaetomium olivaceum via the deletion of a histone deacetylase. Appl Microbiol Biotechnol 108, 332 (2024). https://doi.org/10.1007/s00253-024-13173-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00253-024-13173-8