Abstract
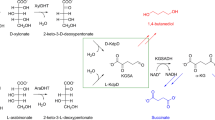
A novel metabolic pathway of 3,6-anhydro-l-galactose (l-AHG), the main sugar component in red macroalgae, was first discovered in the marine bacterium Vibrio sp. EJY3. l-AHG is converted to 2-keto-3-deoxy-galactonate (KDGal) in two metabolic steps. Here, we identified the enantiomeric nature of KDGal in the l-AHG catabolic pathway via stereospecific enzymatic reactions accompanying the biosynthesis of enantiopure l-KDGal and d-KDGal. Enantiopure l-KDGal and d-KDGal were synthesized by enzymatic reactions derived from the fungal galacturonate and bacterial oxidative galactose pathways, respectively. KDGal, which is involved in the l-AHG pathway, was also prepared. The results obtained from the reactions with an l-KDGal aldolase, specifically acting on l-KDGal, showed that KDGal in the l-AHG pathway exists in an l-enantiomeric form. Notably, we demonstrated the utilization of l-KDGal by Escherichia coli for the first time. E. coli cannot utilize l-KDGal as the sole carbon source. However, when a mixture of l-KDGal and d-galacturonate was used, E. coli utilized both. Our study suggests a stereoselective method to determine the absolute configuration of a compound. In addition, our results can be used to explore the novel l-KDGal catabolic pathway in E. coli and to construct an engineered microbial platform that assimilates l-AHG or l-KDGal as substrates.
Key points
• Stereospecific enzyme reactions were used to identify enantiomeric nature of KDGal
• KDGal in the l-AHG catabolic pathway exists in an l-enantiomeric form
• E. coli can utilize l-KDGal as a carbon source when supplied with d-galacturonate







Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information file.
References
Andberg M, Maaheimo H, Boer H, Penttilä M, Koivula A, Richard P (2012) Characterization of a novel Agrobacterium tumefaciens galactarolactone cycloisomerase enzyme for direct conversion of d-galactarolactone to 3-deoxy-2-keto-l-threo-hexarate. J Biol Chem 287:17662–17671
Ashwell, G. (1962) [21] Enzymes of glucuronic and galacturonic acid metabolism in bacteria, Vol. 5: Methods in Enzymology (ed. Academic Press, pp. 190-208).
Deacon J, Cooper RA (1977) d-Galactonate utilisation by enteric bacteria. The catabolic pathway in Escherichia coli. FEBS Lett 77:201–205
Fanood MMR, Ram NB, Lehmann CS, Powis I, Janssen MHM (2015) Enantiomer-specific analysis of multi-component mixtures by correlated electron imaging-ion mass spectrometry. Nat Commun 6:7511
Ha SC, Lee S, Lee J, Kim HT, Ko H-J, Kim KH, Choi I-G (2011) Crystal structure of a key enzyme in the agarolytic pathway, α-neoagarobiose hydrolase from Saccharophagus degradans 2-40. Biochem Biophys Res Commun 412:238–244
Hilditch S, Berghäll S, Kalkkinen N, Penttila M, Richard P (2007) The missing link in the fungal d-galacturonate pathway: identification of the l-threo-3-deoxy-hexulosonate aldolase. J Biol Chem 282:26195–26201
Kim JH, Yun EJ, Seo N, Yu S, Kim DH, Cho KM, An HJ, Kim J-H, Choi I-G, Kim KH (2017) Enzymatic liquefaction of agarose above the sol-gel transition temperature using a thermostable endo-type β-agarase, Aga16B. Appl Microbiol Biotechnol 101:1111–1120
Kuorelahti S, Jouhten P, Maaheimo H, Penttilä M, Richard P (2006) l-galactonate dehydratase is part of the fungal path for d-galacturonic acid catabolism. Mol Microbiol 61:1060–1068
Kuorelahti S, Kalkkinen N, Penttilä M, Londesborough J, Richard P (2005) Identification in the mold Hypocrea jecorina of the first fungal d-galacturonic acid reductase. Biochemistry 44:11234–11240
Kvittingen L, Sjursnes BJ (2020) Demonstrating basic properties and application of polarimetry using a self-constructed polarimeter. J Chem Educ 97:2196–2202
Lagarde AE, Pouysségur JM, Stoeber FR (1973) A transport system for 2-keto-3-deoxy-d-gluconate uptake in Escherichia coli K12. Eur J Biochem 36:328–341
Lee S, Yun EJ, Kim KH, Kim HY, Choi I-G (2017) 3,6-Anhydro-l-galactonate cycloisomerase from Vibrio sp. strain EJY3: crystallization and X-ray crystallographic analysis. Acta Crystallogr F Struct Biol Commun 73:511–514
Parker D (1991) NMR determination of enantiomeric purity. Chem Rev 91:1441–1457
Pouyssegur J, Stoeber F (1974) Genetic control of the 2-keto-3-deoxy-d-gluconate metabolism in Escherichia coli K-12: kdg regulon. J Bacteriol 117:641–651
Seco JM, Quiñoá E, Riguera R (2004) The assignment of absolute configuration by NMR. Chem Rev 104:17–118
Taylor AL, Trotter CD (1972) Linkage map of Escherichia coli strain K-12. Bacteriol Rev 36:504–524
Wiebe MG, Mojzita D, Hilditch S, Ruohonen L, Penttilä M (2010) Bioconversion of d-galacturonate to keto-deoxy-l-galactonate (3-deoxy-l-threo-hex-2-ulosonate) using filamentous fungi. BMC Biotechnol 10:63
Wieczorek SJ, Kalivoda KA, Clifton JG, Ringe D, Petsko GA, Gerlt JA (1999) Evolution of enzymatic activities in the enolase superfamily: identification of a “New” general acid catalyst in the active site of d-galactonate dehydratase from Escherichia coli. J Am Chem Soc 121:4540–4541
Wong TY, Yao XT (1994) The DeLey-Doudoroff pathway of galactose metabolism in Azotobacter vinelandii. Appl Environ Microbiol 60:2065–2068
Yu S, Choi I-G, Yun EJ, Kim KH (2018) High substrate specificity of 3,6-anhydro-l-galactose dehydrogenase indicates its essentiality in the agar catabolism of a marine bacterium. Process Biochem 64:130–135
Yun EJ, Choi I-G, Kim KH (2015a) Red macroalgae as a sustainable resource for bio-based products. Trends Biotechnol 33:247–249
Yun EJ, Lee S, Kim HT, Pelton JG, Kim S, Ko H-J, Choi I-G, Kim KH (2015b) The novel catabolic pathway of 3,6-anhydro-l-galactose, the main component of red macroalgae, in a marine bacterium. Environ Microbiol 17:1677–1688
Yun EJ, Lee S, Kim JH, Kim BB, Kim HT, Lee SH, Pelton JG, Kang NJ, Choi I-G, Kim KH (2013) Enzymatic production of 3,6-anhydro-l-galactose from agarose and its purification and in vitro skin whitening and anti-inflammatory activities. Appl Microbiol Biotechnol 97:2961–2970
Funding
This work was supported by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry, and Fisheries funded by the Ministry of Agriculture, Food, and Rural Affairs (32136-05-1-SB010) and the Ministry of Trade, Industry and Energy (20018132). KHK also received grant supports from Korea University and the Institute of Biomedical Science and Food Safety at the Korea University Food Safety Hall, Korea University. EJY acknowledges a grant support from the National Research Foundation of Korea (RS-2023-00247769).
Author information
Authors and Affiliations
Contributions
EJY and KHK conceived and designed the research. EJY, SY, DHK, and NJP the conducted experiments. EJY and JJL analyzed the data. EJY wrote the manuscript. YSJ and KHK revised the manuscript. All authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Ethics approval
This article does not contain any studies with human participants or animals performed by any authors.
Consent for publication
The authors have consented for publication.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 150 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yun, E.J., Yu, S., Kim, D.H. et al. Identification of the enantiomeric nature of 2-keto-3-deoxy-galactonate in the catabolic pathway of 3,6-anhydro-l-galactose. Appl Microbiol Biotechnol 107, 7427–7438 (2023). https://doi.org/10.1007/s00253-023-12807-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-023-12807-7