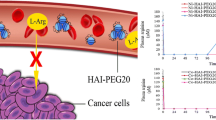
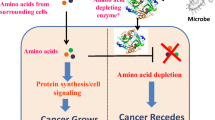
Abstract
Arginine deiminase (ADI) is a microbial-derived enzyme which catalyzes the conversion of l-arginine into l-citrulline. ADI originating from Mycoplasma has been reported to present anti-tumor activity against arginine-auxotrophic tumors, including melanoma. Melanoma cells are sensitive to arginine depletion due to reduced expression of argininosuccinate synthase 1 (ASS1), a key enzyme for arginine biosynthesis. However, clinical applications of recombinant ADI for melanoma treatment present some limitations. Since recombinant ADI is not human-derived, it shows instability, proteolytic degradation, and antigenicity in human serum. In addition, there is a problem of drug resistance issue due to the intracellular expression of once-silenced ASS1. Moreover, recombinant ADI proteins are mainly expressed as inclusion body forms in Escherichia coli and require a time-consuming refolding process to turn them back into active form. Herein, we propose fusion of recombinant ADI from Mycoplasma hominis and 30Kc19α, a cell-penetrating protein which also increases stability and soluble expression of cargo proteins, to overcome these problems. We inserted matrix metalloproteinase-2 cleavable linker between ADI and 30Kc19α to increase enzyme activity in melanoma cells. Compared to ADI, ADI-LK-30Kc19α showed enhanced solubility, stability, and cell penetration. The fusion protein demonstrated selective cytotoxicity and reduced drug resistance in melanoma cells, thus would be a promising strategy for the improved efficacy in melanoma treatment.
Key points
• Fusion of ADI with 30Kc19α enhances soluble expression and productivity of recombinant ADI in E. coli
• 30Kc19α protects ADI from the proteolytic degradation by shielding effect, helping ADI to remain active
• Intracellular delivery of ADI by 30Kc19α overcomes ADI resistance in melanoma cells by degrading intracellularly expressed arginine








Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the article and its supplementary materials.
References
Ahn K-Y, Lee B, Han K-Y, Song J-A, Lee DS, Lee J (2014) Synthesis of Mycoplasma arginine deiminase in E. coli using stress-responsive proteins. Enzyme Microb Technol 63:46–49
Changou CA, Chen Y-R, Xing L, Yen Y, Chuang FY, Cheng RH, Bold RJ, Ann DK, Kung H-J (2014) Arginine starvation-associated atypical cellular death involves mitochondrial dysfunction, nuclear DNA leakage, and chromatin autophagy. Proc Natl Acad Sci USA 111(39):14147–14152
Choi SS, Rhee WJ, Park TH (2005) Beneficial effect of silkworm hemolymph on a CHO cell system: inhibition of apoptosis and increase of EPO production. Biotechnol Bioeng 91(7):793–800
Delage B, Fennell DA, Nicholson L, McNeish I, Lemoine NR, Crook T, Szlosarek PW (2010) Arginine deprivation and argininosuccinate synthetase expression in the treatment of cancer. Int J Cancer 126(12):2762–2772
Dhankhar R, Gupta V, Kumar S, Kapoor RK, Gulati P (2020) Microbial enzymes for deprivation of amino acid metabolism in malignant cells: biological strategy for cancer treatment. Appl Microbiol Biotechnol 104(7):2857–2869
Dillon BJ, Prieto VG, Curley SA, Ensor CM, Holtsberg FW, Bomalaski JS, Clark MA (2004) Incidence and distribution of argininosuccinate synthetase deficiency in human cancers: a method for identifying cancers sensitive to arginine deprivation. Cancer 100(4):826–833
Do BH, Park S, Kwon GG, Nguyen MT, Kang HJ, Song J-A, Yoo J, Nguyen AN, Jang J, Jang M (2017) Soluble expression and purification of bioactive interleukin 33 in E coli. Biotechnol Bioprocess Eng 22(3):256–264
El-Sayed AS, Shindia AA, Abou Zeid AA, Yassin AM, Sitohy MZ, Sitohy B (2019) Aspergillus nidulans thermostable arginine deiminase-Dextran conjugates with enhanced molecular stability, proteolytic resistance, pharmacokinetic properties and anticancer activity. Enzyme Microb Technol 131:109432
Ensor CM, Holtsberg FW, Bomalaski JS, Clark MA (2002) Pegylated arginine deiminase (ADI-SS-PEG 20,000 MW) inhibits human melanomas and hepatocellular carcinomas in vitro and in vivo. Cancer Res 62(19):5443–5450
Fayura LR, Boretsky YR, Pynyaha YV, Wheatley DN, Sibirny AA (2013) Improved method for expression and isolation of the Mycoplasma hominis arginine deiminase from the recombinant strain of Escherichia coli. J Biotech 167(4):420–426
Gasteiger E, Hoogland C, Gattiker A, Wilkins MR, Appel RD, Bairoch A (2005) Protein identification and analysis tools on the ExPASy server. In: Walker JM (ed) The proteomics protocols handbook. Springer, pp 571–607
Hall PE, Lewis R, Syed N, Shaffer R, Evanson J, Ellis S, Williams M, Feng X, Johnston A, Thomson JA (2019) A phase I study of pegylated arginine deiminase (Pegargiminase), cisplatin, and pemetrexed in argininosuccinate synthetase 1-deficient recurrent high-grade glioma arginine deprivation therapy in recurrent high-grade gliomas. Clin Cancer Res 25(9):2708–2716
Hussain Z, Khan S, Imran M, Sohail M, Shah SWA, de Matas M (2019) PEGylation: A promising strategy to overcome challenges to cancer-targeted nanomedicines: a review of challenges to clinical transition and promising resolution. Drug Deliv Transl Res 9(3):721–734
Husson A, Brasse-Lagnel C, Fairand A, Renouf S, Lavoinne A (2003) Argininosuccinate synthetase from the urea cycle to the citrulline–NO cycle. Eur J Biochem 270(9):1887–1899
Ji JX, Cochrane DR, Tessier-Cloutier B, Chen SY, Ho G, Pathak KV, Alcazar IN, Farnell D, Leung S, Cheng A (2020) Arginine depletion therapy with ADI-PEG20 limits tumor growth in argininosuccinate synthase–deficient ovarian cancer, including small-cell carcinoma of the ovary, hypercalcemic type arginine depletion to treat rare ovarian cancers. Clin Cancer Res 26(16):4402–4413
Jobin PG, Butler GS (1864) Overall CM (2017) New intracellular activities of matrix metalloproteinases shine in the moonlight. Biochim Biophys Acta - Mol 11:2043–2055
Kang Y-S, Song J-A, Han K-Y, Lee J (2015) Escherichia coli EDA is a novel fusion expression partner to improve solubility of aggregation-prone heterologous proteins. J Biotechnol 194:39–47
Kawatra A, Dhankhar R, Gulati P (2021) Microbial arginine deiminase: a multifaceted green catalyst in biomedical sciences. Int J Biol Macromol 196:151–162
Kim EJ, Park TH (2003) Anti-apoptosis engineering. Biotechnol. Bioprocess Eng 8(2):76–82
Kim EJ, Rhee WJ, Park TH (2004) Inhibition of apoptosis by a Bombyx mori gene. Biotechnol Prog 20(1):324–329
Kim JE, Kim EJ, Rhee WJ, Park TH (2005) Enhanced production of recombinant protein in Escherichia coli using silkworm hemolymph. Biotechnol Bioprocess Eng 10(4):353
Kim RH, Coates JM, Bowles TL, McNerney GP, Sutcliffe J, Jung JU, Gandour-Edwards R, Chuang FY, Bold RJ, Kung H-J (2009) Arginine deiminase as a novel therapy for prostate cancer induces autophagy and caspase-independent apoptosis. Cancer Res 69(2):700–708
Kim SHL, Cho S, Kim S, Kwon J, Lee J, Koh RH, Park JH, Lee H, Park TH, Hwang NS (2022) Cellular direct conversion by cell penetrable OCT4-30Kc19 protein and BMP4 growth factor. Biomater Res 26(1):1–18
Lee H, An YH, Kim TK, Ryu J, Park GK, Park MJ, Ko J, Kim H, Choi HS, Hwang NS (2021) Enhancement of wound healing efficacy by increasing the stability and skin-penetrating property of bFGF using 30Kc19α-based fusion protein. Adv Biol 5(1):2000176
Lee H, Kim SHL, Yoon H, Ryu J, Park HH, Hwang NS, Park TH (2020) Intracellular delivery of recombinant RUNX2 facilitated by cell-penetrating protein for the osteogenic differentiation of hMSCs. ACS Biomater Sci Eng 6(9):5202–5214
Lee HJ, Park HH, Kim JA, Park JH, Ryu J, Choi J, Lee J, Rhee WJ, Park TH (2014) Enzyme delivery using the 30Kc19 protein and human serum albumin nanoparticles. Biomater 35(5):1696–1704
Lindgren M, Hällbrink M, Prochiantz A, Langel Ü (2000) Cell-penetrating peptides. Trends Pharmacol Sci 21(3):99–103
Long Y, Tsai W-B, Wangpaichitr M, Tsukamoto T, Savaraj N, Feun LG, Kuo MT (2013) Arginine deiminase resistance in melanoma cells is associated with metabolic reprogramming, glucose dependence, and glutamine addiction. Mol Cancer Ther 12(11):2581–2590
Mäe M, Langel Ü (2006) Cell-penetrating peptides as vectors for peptide, protein and oligonucleotide delivery. Curr Opin Pharmacol 6(5):509–514
Mishra P, Nayak B, Dey R (2016) PEGylation in anti-cancer therapy: an overview. Asian J Pharm Sci 11(3):337–348
Miyazaki K, Takaku H, Umeda M, Fujita T, Huang W, Kimura T, Yamashita J, Horio T (1990) Potent growth inhibition of human tumor cells in culture by arginine deiminase purified from a culture medium of a Mycoplasma-infected cell line. Cancer Res 50(15):4522–4527
Ni Y, Schwaneberg U, Sun Z-H (2008) Arginine deiminase, a potential anti-tumor drug. Cancer Lett 261(1):1–11
Oginsky EL (1957) Isolation and determination of arginine and citrulline. Methods Enzymol 3:639–643
Oh-Hashi K, Furuta E, Fujimura K, Hirata Y (2017) Application of a novel HiBiT peptide tag for monitoring ATF4 protein expression in Neuro2a cells. Biochem Biophys Rep 12:40–45
Phan NM, Nguyen TL, Kim J (2022) Nanozyme-based enhanced cancer immunotherapy. Tissue Eng Regen Med 19(2):237–252
Park G (2022) Enhancement of solubility, cell penetration, and stability of arginine deiminase using 30Kc19α for effective melanoma treatment. Dissertation, Seoul National University
Park HH, Sohn Y, Yeo JW, Park JH, Lee HJ, Ryu J, Rhee WJ, Park TH (2014a) Dimerization of 30Kc19 protein in the presence of amphiphilic moiety and importance of Cys-57 during cell penetration. Biotechnol J 9(12):1582–1593
Park HH, Sohn Y, Yeo JW, Park JH, Lee HJ, Ryu J, Rhee WJ, Park TH (2014b) Identification and characterization of a novel cell-penetrating peptide of 30Kc19 protein derived from Bombyx mori. Process Biochem 49(9):1516–1526
Park HH, Woo YH, Ryu J, Lee HJ, Park JH, Park TH (2017) Enzyme delivery using protein-stabilizing and cell-penetrating 30Kc19α protein nanoparticles. Process Biochem 63:76–83
Park JH, Lee JH, Park HH, Rhee WJ, Choi SS, Park TH (2012a) A protein delivery system using 30Kc19 cell-penetrating protein originating from silkworm. Biomater 33(35):9127–9134
Park JH, Park HH, Choi SS, Park TH (2012b) Stabilization of enzymes by the recombinant 30Kc19 protein. Process Biochem 47(1):164–169
Patil M, Bhaumik J, Babykutty S, Banerjee U, Fukumura D (2016) Arginine dependence of tumor cells: targeting a chink in cancer’s armor. Oncogene 35(38):4957–4972
Pereira AMM, Strasberg-Rieber M, Rieber M (2005) Invasion-associated MMP-2 and MMP-9 are up-regulated intracellularly in concert with apoptosis linked to melanoma cell detachment. Clin Exp Metastasis 22(4):285–295
Phillips MM, Sheaff MT, Szlosarek PW (2013) Targeting arginine-dependent cancers with arginine-degrading enzymes: opportunities and challenges. Cancer Res Treat 45(4):251
Redondo P, Lloret P, Idoate M, Inoges S (2005) Expression and serum levels of MMP-2 and MMP-9 during human melanoma progression. Clin Exp Dermatol 30(5):541–545
Rhee WJ, Kim EJ, Park TH (1999) Kinetic effect of silkworm hemolymph on the delayed host cell death in an insect cell-baculovirus system. Biotechnol Prog 15(6):1028–1032
Rhee WJ, Lee EH, Park TH (2009) Expression of Bombyx mori 30Kc19 protein in Escherichia coli and its anti-apoptotic effect in Sf9 cell. Biotechnol Bioprocess Eng 14(5):645
Riess C, Shokraie F, Classen CF, Kreikemeyer B, Fiedler T, Junghanss C, Maletzki C (2018) Arginine-depleting enzymes–an increasingly recognized treatment strategy for therapy-refractory malignancies. Cell Physiol Biochem 51(2):854–870
Roomi M, Monterrey J, Kalinovsky T, Rath M, Niedzwiecki A (2009) Patterns of MMP-2 and MMP-9 expression in human cancer cell lines. Oncol Rep 21(5):1323–1333
Ryu J, Hwang NS, Park HH, Park TH (2020) Protein-based direct reprogramming of fibroblasts to neuronal cells using 30Kc19 protein and transcription factor Ascl1. Int J Biochem Cell Biol 121:105717
Ryu J, Kim H, Park HH, Lee HJ, Park JH, Rhee WJ, Park TH (2016a) Protein-stabilizing and cell-penetrating properties of α-helix domain of 30Kc19 protein. Biotechnol J 11(11):1443–1451
Ryu J, Park HH, Park JH, Lee HJ, Rhee WJ, Park TH (2016b) Soluble expression and stability enhancement of transcription factors using 30Kc19 cell-penetrating protein. Appl Microbiol Biotechnol 100(8):3523–3532
Schneier M, Razdan S, Miller AM, Briceno ME, Barua S (2020) Current technologies to endotoxin detection and removal for biopharmaceutical purification. Biotechnol Bioeng 117(8):2588–2609
Singh SM, Panda AK (2005) Solubilization and refolding of bacterial inclusion body proteins. J Biosci Bioeng 99(4):303–310
Solli AI, Fadnes B, Winberg J-O, Uhlin-Hansen L, Hadler-Olsen E (2013) Tissue-and cell-specific co-localization of intracellular gelatinolytic activity and matrix metalloproteinase 2. J Histochem Cytochem 61(6):444–461
Son B, Yoon H, Ryu J, Lee H, Joo J, Park HH, Park TH (2022) Enhanced efficiency of generating human-induced pluripotent stem cells using Lin28-30Kc19 fusion protein. Front Bioeng Biotechnol 10:911614
Song J-A, Lee D-S, Park J-S, Han K-Y, Lee J (2011) A novel Escherichia coli solubility enhancer protein for fusion expression of aggregation-prone heterologous proteins. Enzyme Microb Technol 49(2):124–130
Sun N, Zhao X (2022) Argininosuccinate synthase 1, arginine deprivation therapy and cancer management. Front Pharmacol 13:935553
Tsai W-B, Aiba I, Lee S-y, Feun L, Savaraj N, Kuo MT (2009) Resistance to arginine deiminase treatment in melanoma cells is associated with induced argininosuccinate synthetase expression involving c-Myc/HIF-1α/Sp4. Mol Cancer Ther 8(12):3223–3233
Wu F-LL, Yeh T-H, Chen Y-L, Chiu Y-C, Cheng J-C, Wei M-F, Shen L-J (2014) Intracellular delivery of recombinant arginine deiminase (rADI) by heparin-binding hemagglutinin adhesion peptide restores sensitivity in rADI-resistant cancer cells. Mol Pharm 11(8):2777–2786
Yang J-P, Ma X-X, He Y-X, Li W-F, Kang Y, Bao R, Chen Y, Zhou C-Z (2011) Crystal structure of the 30 K protein from the silkworm Bombyx mori reveals a new member of the β-trefoil superfamily. J Struct Biol 175(1):97–103
Yao Q, Kou L, Tu Y, Zhu L (2018) MMP-responsive ‘smart’ drug delivery and tumor targeting. Trends Pharmacol Sci 39(8):766–781
Yeh T-H, Chen Y-R, Chen S-Y, Shen W-C, Ann DK, Zaro JL, Shen L-J (2016) Selective intracellular delivery of recombinant arginine deiminase (ADI) using pH-sensitive cell penetrating peptides to overcome ADI resistance in hypoxic breast cancer cells. Mol Pharm 13(1):262–271
Yu K, Liu C, Kim B-G, Lee D-Y (2015) Synthetic fusion protein design and applications. Biotechnol Adv 33(1):155–164
Zhang F, Liu M-r, Wan H-t (2014) Discussion about several potential drawbacks of PEGylated therapeutic proteins. Biol Pharm Bull 37(3):335–339
Zúñiga M, Pérez G, González-Candelas F (2002) Evolution of arginine deiminase (ADI) pathway genes. Mol Phylogenetics Evol 25(3):429–444
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2020R1A4A3078645, 2021R1C1C1014606).
Author information
Authors and Affiliations
Contributions
HL and GP collected data and evidence. HHP and THP conceived and designed the study. HL, GP, and HHP wrote the manuscript. HL drew the figures. HL, GP, SK, BS, JJ, and HHP directed and validated the data analysis. All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lee, H., Park, G., Kim, S. et al. Enhancement of anti-tumor activity in melanoma using arginine deiminase fused with 30Kc19α protein. Appl Microbiol Biotechnol 106, 7531–7545 (2022). https://doi.org/10.1007/s00253-022-12218-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-022-12218-0