Abstract
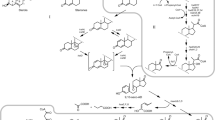
Modified β-cyclodextrins are widely used for the enhancement of microbial conversions of lipophilic compounds such as steroids. Multiple mechanisms of cyclodextrin-mediated enhancement of phytosterol bioconversion by mycobacteria had previously been shown to include steroid solubilization, alterations in the cell wall permeability for both steroids and nutrients, facilitation of protein leaking, and activity suppression of some steroid-transforming enzymes.In this work, we studied whether cyclodextrins might affect expression of the genes involved in the steroid catabolic pathway. Phytosterol bioconversion with 9α-hydroxy-androst-4-ene-3,17-dione accumulation by Mycobacterium sp. VKM Ac-1817D in the presence of methylated β-cyclodextrin (MCD) was investigated. RNA sequencing of the whole transcriptomes in different combinations of phytosterol and MCD showed a similar expression level of the steroid catabolism genes related to the KstR-regulon and was responsible for side chain and initial steps of steroid core oxidation; whereas, induction levels of the genes related to the KstR2-regulon were attenuated in the presence of MCD in this strain. The data were attenuated with quantitative real-time PCR.The results contribute to the understanding of cyclodextrin effects on microbial steroid conversion and provide a basis for the use of cyclodextrins as expression enhancers for studies of sterol catabolism in actinobacteria.



Similar content being viewed by others
References
Bragin EY, Shtratnikova VY, Dovbnya DV, Schelkunov MI, Pekov YA, Malakho SG, Egorova OV, Ivashina TV, Sokolov SL, Ashapkin VV, Donova MV (2013) Comparative analysis of genes encoding key steroid core oxidation enzymes in fast-growing Mycobacterium spp. strains. J Steroid Biochem Mol Biol 138:41–53. doi:10.1016/j.jsbmb.2013.02.016
Bragin EY, Shtratnikova VY, Dovbnya DV, Schelkunov MI, Donova MV (2015) Identification of sitosterol-induced genes encoding steroid nucleus rings C and D degradation in sterol transforming strains mycobacteria. Russ J Immunol 9:582–583
Casabon I, Crowe AM, Liu J, Eltis LD (2013) FadD3 is an acyl-CoA synthetase that initiates catabolism of cholesterol rings C and D in actinobacteria: role of FadD3 in cholesterol catabolism. Mol Microbiol 87:269–283. doi:10.1111/mmi.12095
Conesa A, Götz S, García-Gómez JM, Terol J, Talón M, Robles M (2005) Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinforma Oxf Engl 21:3674–3676. doi:10.1093/bioinformatics/bti610
Crowe AM, Stogios PJ, Casabon I, Evdokimova E, Savchenko A, Eltis LD (2015) Structural and functional characterization of a ketosteroid transcriptional regulator of Mycobacterium tuberculosis. J Biol Chem 290:872–882. doi:10.1074/jbc.M114.607481
Donova MV, Dovbnya DV, Sukhodolskaya GV, Khomutov SM, Nikolayeva VM, Kwon I, Han K (2005) Microbial conversion of sterol-containing soybean oil production waste. J Chem Technol Biotechnol 80:55–60. doi:10.1002/jctb.1156
Donova MV, Nikolayeva VM, Dovbnya DV, Gulevskaya SA, Suzina NE (2007) Methyl-β-cyclodextrin alters growth, activity and cell envelope features of sterol-transforming mycobacteria. Microbiology 153:1981–1992. doi:10.1099/mic.0.2006/001636-0
Dovbnya DV, Khomutov SM, Nikolayeva VM, Donova MV (1999) CD-medium control of microbial sterol sidechain cleavage. In: Torres Labandeira JJ, Vila-Jato JL (eds) Proc Ninth Internat Symp on Cyclodextrins. Springer Netherlands, Dordrecht, pp 395–398. doi:10.1007/978-94-011-4681-4
Hesselink P, Vliet S, Vries H, Witholt B (1989) Optimization of steroid side chain deavage by Mycobacterium sp. in the presence of cyclodextrins. Enzym Microb Technol 11:398–404
Horinouchi M, Kurita T, Yamamoto T, Hatori E, Hayashi T, Kudo T (2004) Steroid degradation gene cluster of Comamonas testosteroni consisting of 18 putative genes from meta-cleavage enzyme gene tesB to regulator gene tesR. Biochem Biophys Res Commun 324:597–604. doi:10.1016/j.bbrc.2004.09.096
Horinouchi M, Hayashi T, Kudo T (2012) Steroid degradation in Comamonas testosteroni. J Steroid Biochem Mol Biol 129:4–14. doi:10.1016/j.jsbmb.2010.10.008
Jadoun J, Bar R (1993a) Microbial transformations in a cyclodextrin medium. Part 3. Cholesterol oxidation by Rhodococcus erythropolis. Appl Microbiol Biotechnol 40:230–240. doi:10.1007/BF00170372
Jadoun J, Bar R (1993b) Microbial transformations in a cyclodextrin medium. Part 4. Enzyme vs microbial oxidation of cholesterol. Appl Microbiol Biotechnol 40:477–482. doi:10.1007/BF00175734
Kendall SL, Withers M, Soffair CN, Moreland NJ, Gurcha S, Sidders B, Frita R, Ten Bokum A, Besra GS, Lott JS, Stoker NG (2007) A highly conserved transcriptional repressor controls a large regulon involved in lipid degradation in Mycobacterium smegmatis and Mycobacterium tuberculosis: transcriptional repressor controlling a large lipid metabolism regulon in mycobacteria. Mol Microbiol 65:684–699. doi:10.1111/j.1365-2958.2007.05827.x
Kendall SL, Burgess P, Balhana R, Withers M, ten Bokum A, Lott JS, Gao C, Uhia-Castro I, Stoker NG (2010) Cholesterol utilization in mycobacteria is controlled by two TetR-type transcriptional regulators: kstR and kstR2. Microbiology 156:1362–1371. doi:10.1099/mic.0.034538-0
Khomutov SM, Sidorov IA, Dovbnya DV, Donova MV (2002) Estimation of cyclodextrin affinity to steroids. J Pharm Pharmacol 54:617–622
Khomutov SM, Sukhodolskaya GV, Donova MV (2007) The inhibitory effect of cyclodextrin on the degradation of 9α-hydroxyandrost-4-ene-3,17-dione by Mycobacterium sp. VKM Ac-1817D. Biocatal Biotransformation 25:386–392. doi:10.1080/10242420701510510
Li L, Stoeckert CJ, Roos DS (2003) OrthoMCL: identification of ortholog groups for eukaryotic genomes. Genome Res 13:2178–2189. doi:10.1101/gr.1224503
Li F, Zhu L, Wang L, Zhan Y (2015) Gene expression of an arthrobacter in surfactant-enhanced biodegradation of a hydrophobic organic compound. Environ Sci Technol 49:3698–3704. doi:10.1021/es504673j
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods San Diego Calif 25:402–408. doi:10.1006/meth.2001.1262
López-Nicolás JM, Bru R, Garcı́a-Carmona F (1997) Kinetic characteristics of the enzymatic conversion in presence of cyclodextrins: study of the oxidation of polyunsaturated fatty acids by lipoxygenase. Biochimica et Biophysica Acta (BBA) - Lipids and Lipid Metabolism 1347 (2-3):140–150
Ma Y-H, Wang M, Fan Z, Shen Y-B, Zhang L-T (2009) The influence of host-guest inclusion complex formation on the biotransformation of cortisone acetate Δ1-dehydrogenation. J Steroid Biochem Mol Biol 117:146–151. doi:10.1016/j.jsbmb.2009.08.007
Mohn WW, Wilbrink MH, Casabon I, Stewart GR, Liu J, van der Geize R, Eltis LD (2012) Gene cluster encoding cholate catabolism in Rhodococcus spp. J Bacteriol 194:6712–6719. doi:10.1128/JB.01169-12
Petrusma M, Hessels G, Dijkhuizen L, van der Geize R (2011) Multiplicity of 3-ketosteroid-9α-hydroxylase enzymes in Rhodococcus rhodochrous DSM43269 for specific degradation of different classes of steroids. J Bacteriol 193:3931–3940. doi:10.1128/JB.00274-11
Rocha DJP, Santos CS, Pacheco LGC (2015) Bacterial reference genes for gene expression studies by RT-qPCR: survey and analysis. Antonie Van Leeuwenhoek 108:685–693. doi:10.1007/s10482-015-0524-1
Roglič U, Žnidaršič-Plazl P, Plazl I (2005) The influence of β-cyclodextrin on the kinetics of progesterone transformation by Rhizopus nigricans. Biocatal Biotransformation 23:299–305. doi:10.1080/10242420500175929
Shen Y, Wang M, Zhang L, Ma Y, Ma B, Zheng Y, Liu H, Luo J (2011) Effects of hydroxypropyl-β-cyclodextrin on cell growth, activity, and integrity of steroid-transforming Arthrobacter simplex and Mycobacterium sp. Appl Microbiol Biotechnol 90:1995–2003. doi:10.1007/s00253-011-3214-6
Shen Y-B, Wang M, Li H-N, Wang Y-B, Luo J-M (2012) Influence of hydroxypropyl-β-cyclodextrin on phytosterol biotransformation by different strains of Mycobacterium neoaurum. J Ind Microbiol Biotechnol 39:1253–1259. doi:10.1007/s10295-012-1130-0
Shtratnikova VY, Schelkunov MI, Dovbnya DV, Pekov YA, Bragin EY, Ashapkin VV, Donova MV (2015) Complete genome sequence of Mycobacterium sp. strain VKM Ac-1817D, capable of producing 9α-hydroxy-androst-4-ene-3,17-dione from phytosterol. Genome Announc 3:e01447–e01414. doi:10.1128/genomeA.01447-14
Shtratnikova VY, Schelkunov MI, Fokina VV, Pekov YA, Ivashina T, Donova MV (2016) Genome-wide bioinformatics analysis of steroid metabolism-associated genes in Nocardioides simplex VKM Ac-2033D. Curr Genet 62:643–656. doi:10.1007/s00294-016-0568-4
Singer Y, Shity H, Bar R (1991) Microbial transformations in a cyclodextrin medium. Part 2. Reduction of androstenedione to testosterone by Saccharomyces cerevisiae. Appl Microbiol Biotechnol 35:731–737. doi:10.1007/BF00169886
Sukhodolskaya GV, Nikolayeva VM, Khomutov SM, Donova MV (2007) Steroid-1-dehydrogenase of Mycobacterium sp. VKM Ac-1817D strain producing 9α-hydroxy-androst-4-ene-3,17-dione from sitosterol. Appl Microbiol Biotechnol 74:867–873. doi:10.1007/s00253-006-0728-4
Szejtli J (1997) Utilization of cyclodextrins in industrial products and processes. J Mater Chem 7:575–587. doi:10.1039/a605235e
Szentirmai A (1990) Microbial physiology of side chain degradation of steroids. J Ind Microbiol Biotechnol 6:101–115. doi:10.1007/BF01576429
Tang H, Klopfenstein D, Pedersen B, Flick P, Sato K, Ramirez F, Yunes J, Mungall C (2015) GOATOOLS: tools for gene ontology. Zenodo. doi:10.5281/zenodo.31628
Thomas ST, Sampson NS (2013) Mycobacterium tuberculosis utilizes a unique heterotetrameric structure for dehydrogenation of the cholesterol side chain. Biochemistry 52:2895–2904. doi:10.1021/bi4002979
Tjaden B (2015) De novo assembly of bacterial transcriptomes from RNA-seq data. Genome Biol 16:1. doi:10.1186/s13059-014-0572-2
Uhía I, Galán B, Morales V, García JL (2011) Initial step in the catabolism of cholesterol by Mycobacterium smegmatis mc2 155: cholesterol catabolism in Mycobacterium smegmatis. Environ Microbiol 13:943–959. doi:10.1111/j.1462-2920.2010.02398.x
Uhía I, Galán B, Kendall SL, Stoker NG, García JL (2012) Cholesterol metabolism in Mycobacterium smegmatis: cholesterol pathway. Environ Microbiol Rep 4:168–182. doi:10.1111/j.1758-2229.2011.00314.x
Van der Geize R, Yam K, Heuser T, Wilbrink MH, Hara H, Anderton MC, Sim E, Dijkhuizen L, Davies JE, Mohn WW, Eltis LD (2007) A gene cluster encoding cholesterol catabolism in a soil actinomycete provides insight into Mycobacterium tuberculosis survival in macrophages. Proc Natl Acad Sci U S A 104:1947–1952. doi:10.1073/pnas.0605728104
Van der Geize R, Grommen AWF, Hessels GI, Jacobs AC, Dijkhuizen L (2011) The steroid catabolic pathway of the intracellular pathogen Rhodococcus equi is important for pathogenesis and a target for vaccine development. PLoS Pathog 7:e1002181. doi:10.1371/journal.ppat.1002181
Wilbrink MH (2011) Microbial sterol side chain degradation in actinobacteria. University Library Groningen, Groningen
Wilbrink MH, van der Geize R, Dijkhuizen L (2012) Molecular characterization of ltp3 and ltp4, essential for C24-branched chain sterol-side-chain degradation in Rhodococcus rhodochrous DSM 43269. Microbiology 158:3054–3062. doi:10.1099/mic.0.059501-0
Wipperman MF, Yang M, Thomas ST, Sampson NS (2013) Shrinking the FadE proteome of Mycobacterium tuberculosis: insights into cholesterol metabolism through identification of an α2β2 heterotetrameric acyl coenzyme A dehydrogenase family. J Bacteriol 195:4331–4341. doi:10.1128/JB.00502-13
Acknowledgements
This work was supported by a Russian Science Foundation (Grant No. 14-24-00169).
Author information
Authors and Affiliations
Contributions
VS and EB designed and carried out mRNA isolation, high-throughput sequencing, RT-PCR experiments, and data analysis; MS made bioinformatics calculations; DD designed and carried out growth, bioconversion, and induction experiments; VS, MS, DD, and MD wrote the manuscript; MD coordinated the project, participated in the conception, drafting, and revision of the manuscript. All authors read and approved the final manuscript before submission.
Corresponding author
Ethics declarations
Funding
This work was supported by a Russian Science Foundation (Grant No. 14-24-00169).
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Shtratnikova, V.Y., Schelkunov, M.I., Dovbnya, D.V. et al. Effect of methyl-β-cyclodextrin on gene expression in microbial conversion of phytosterol. Appl Microbiol Biotechnol 101, 4659–4667 (2017). https://doi.org/10.1007/s00253-017-8288-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-017-8288-3