Abstract
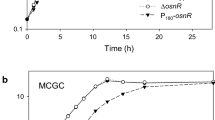
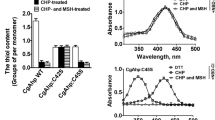
Corynebacterium glutamicum ORF NCgl0328, designated noxA, encodes an NADH oxidase enzyme. The noxA gene, which was preferentially expressed in the log growth phase, was found to be under the control of the whcA, whcB, and whcE genes, which play regulatory roles in cells under oxidative stress. While noxA transcription was minimal in whcE-deleted mutant cells (ΔwhcE) during growth, its transcription was maximal even in the stationary phase in ΔwhcA cells. The transcription levels of noxA in ΔwhcB and whcB-overexpressing cells were comparable to the levels only in the log growth phase in ΔwhcA and whcA-overexpressing cells, respectively. Direct binding of purified WhcA to the promoter region of noxA was observed in vitro. The DNA-protein interaction was only possible in the presence of the reducing agent dithiothreitol. A noxA-deleted mutant strain and a strain overexpressing the noxA gene (P180-noxA) were established, and these strains were found to exhibit defective cell growth. The ΔnoxA and P180-noxA strains were sensitive to the redox-cycling oxidant menadione, suggesting a role of noxA in redox balancing. Accordingly, the purified NoxA enzyme exhibited NADH-oxidizing activity. Taken together, these data show that noxA plays a role in oxidative stress responses and also that the gene is under direct control of the WhcA protein, which was shown to be a regulatory DNA-binding protein. Furthermore, the involvement and roles of the whcA, whcB, and whcE genes in regulating the expression of noxA were demonstrated.





Similar content being viewed by others
References
Afanas’ev IB, Korkina LG, Suslova TB, Soodaeva SK (1990) Are quinones producers or scavengers of superoxide ion in cells? Arch Biochem Biophys 281:245–250
Alam MS, Garg SK, Agrawal P (2007) Molecular function of WhiB4/Rv3681c of Mycobacterium tuberculosis H37Rv: a [4Fe−4S] cluster coordinating protein disulphide reductase. Mol Microbiol 63:1414–1431
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chenier D, Beriault R, Mailloux R, Baquie M, Abramia G, Lemire J, Appanna V (2008) Involvement of fumarase C and NADH oxidase in metabolic adaptation of Pseudomonas fluorescens cells evoked by aluminum and gallium toxicity. Appl Environ Microbiol 74:3977–3984
Choi WW, Park SD, Lee SM, Kim HB, Kim Y, Lee HS (2009) The whcA gene plays a negative role in oxidative stress response of Corynebacterium glutamicum. FEMS Microbiol Lett 290:32–38
Cortial S, Chaignon P, Iorga BI, Aymerich S, Truan G, Gueguen-Chaignon V, Meyer P, Moréra S, Ouazzani (2010) NADH oxidase activity of Bacillus subtilis nitroreductase NfrA1: insight into its biological role. FEBS Lett 584:3916–3922
Crack JC, den Hengst CD, Jakimowicz P, Subramanian S, Johnson MK, Buttner MJ, Thomson AJ, Le Brun NE (2009) Characterization of [4Fe-4S]-containing and cluster-free forms of Streptomyces WhiD. Biochemistry (NY) 48:12252–12264
Davis NK, Chater KF (1992) The Streptomyces coelicolor whiB gene encodes a small transcription factor-like protein dispensable for growth but essential for sporulation. Mol Gen Genet MGG 232:351–358
Derr AM, Faustoferri RC, Betzenhauser MJ, Gonzalez K, Marquis RE, Quivey RG Jr (2012) Mutation of the NADH oxidase gene (nox) reveals an overlap of the oxygen- and acid-mediated stress responses in Streptococcus mutans. Appl Environ Microbiol 78:1215–1227
Follettie M, Sinskey A (1986) Recombinant DNA technology for Corynebacterium glutamicum. Food Technol 40:88–94
Follettie MT, Peoples O, Agoropoulou C, Sinskey A (1993) Gene structure and expression of the Corynebacterium flavum N13 ask-asd operon. J Bacteriol 175:4096–4103
Gao B, Gupta RS (2012) Phylogenetic framework and molecular signatures for the main clades of the phylum Actinobacteria. Microbiol Mol Biol Rev 76:66–112
Holmgren A (1985) Thioredoxin. Annu Rev Biochem 54:237–271
Hong EJ, Park JS, Kim Y, Lee HS (2014) Role of Corynebacterium glutamicum sprA encoding a serine protease in glxR-mediated global gene regulation. PLoS One 9:e93587
Hou J, Suo F, Wang C, Li X, Shen Y, Bao X (2014) Fine-tuning of NADH oxidase decreases byproduct accumulation in respiration deficient xylose metabolic Saccharomyces cerevisiae. BMC Biotechnol 14:13
Hwang BJ, Yeom HJ, Kim Y, Lee HS (2002) Corynebacterium glutamicum utilizes both transsulfuration and direct sulfhydrylation pathways for methionine biosynthesis. J Bacteriol 184:1277–1286
Ikeda M, Nakagawa S (2003) The Corynebacterium glutamicum genome: features and impacts on biotechnological processes. Appl Microbiol Biotechnol 62:99–109
Jakimowicz P, Cheesman MR, Bishai WR, Chater KF, Thomson AJ, Buttner MJ (2005) Evidence that the Streptomyces developmental protein WhiD, a member of the WhiB family, binds a [4Fe-4S] cluster. J Biol Chem 280:8309–8315
Ji XJ, Xia ZF, Fu NH, Nie ZK, Shen MQ, Tian QQ, Huang H (2013) Cofactor engineering through heterologous expression of an NADH oxidase and its impact on metabolic flux redistribution in Klebsiella pneumoniae. Biotechnol Biofuels 6:7
Jia B, Lee S, Pham BP, Cho YS, Yang J, Byeon H, Kim JC, Cheong G (2010) An archaeal NADH oxidase causes damage to both proteins and nucleic acids under oxidative stress. Mol Cells 29:363–371
Kalinowski J, Bathe B, Bartels D, Bischoff N, Bott M, Burkovski A, Dusch N, Eggeling L, Eikmanns BJ, Gaigalat L, Goesmann A, Hartmann M, Huthmacher K, Krämer R, Linke B, McHardy AC, Meyer F, Möckel B, Pfefferle W, Pühler A, Rey DA, Rückert C, Rupp O, Sahm H, Wendisch VF, Wiegräbe I, Tauch A (2003) The complete Corynebacterium glutamicum ATCC 13032 genome sequence and its impact on the production of L-aspartate-derived amino acids and vitamins. J Biotechnol 104:5–25
Kang TS, Korber DR, Tanaka T (2013) Influence of oxygen on NADH recycling and oxidative stress resistance systems in Lactobacillus panis PM1. AMB Expr 3:1–9
Kawasaki S, Watamura Y, Ono M, Watanabe T, Takeda K, Niimura Y (2005) Adaptive responses to oxygen stress in obligatory anaerobes Clostridium acetobutylicum and Clostridium aminovalericum. Appl Environ Microbiol 71:8442–8450
Kawasaki S, Satoh T, Todoroki M, Niimura Y (2009) b-type dihydroorotate dehydrogenase is purified as a H2O2-forming NADH oxidase from Bifidobacterium bifidum. Appl Environ Microbiol 75:629–636
Kim HJ, Kim TH, Kim Y, Lee HS (2004) Identification and characterization of glxR, a gene involved in regulation of glyoxylate bypass in Corynebacterium glutamicum. J Bacteriol 186:3453–3460
Kim TH, Park JS, Kim HJ, Kim Y, Kim P, Lee HS (2005) The whcE gene of Corynebacterium glutamicum is important for survival following heat and oxidative stress. Biochem Biophys Res Commun 337:757–764
Kundu TK, Velayutham M, Zweier JL (2012) Aldehyde oxidase functions as a superoxide generating NADH oxidase: an important redox regulated pathway of cellular oxygen radical formation. Biochemistry 51:2930–2939
Lee JY, Park JS, Kim HJ, Kim Y, Lee HS (2012) Corynebacterium glutamicum whcB, a stationary phase‐specific regulatory gene. FEMS Microbiol Lett 327:103–109
Lee JY, Kim HJ, Kim ES, Kim P, Kim Y, Lee HS (2013) Regulatory interaction of the Corynebacterium glutamicum whc genes in oxidative stress responses. J Biotechnol 168:149–154
Leuchtenberger W, Huthmacher K, Drauz K (2005) Biotechnological production of amino acids and derivatives: current status and prospects. Appl Microbiol Biotechnol 69:1–8
MacNeil DJ, Gewain KM, Ruby CL, Dezeny G, Gibbons PH, MacNeil T (1992) Analysis of Streptomyces avermitilis genes required for avermectin biosynthesis utilizing a novel integration vector. Gene 111:61–68
Park SD, Lee SN, Park IY, Choi JS, Jeong WK, Kim Y, Lee HS (2004) Isolation and characterization of transcriptional elements from Corynebacterium glutamicum. J Microbiol Biotechnol 14:789–795
Park SD, Youn JW, Kim YJ, Lee SM, Kim Y, Lee HS (2008) Corynebacterium glutamicum σE is involved in responses to cell surface stresses and its activity is controlled by the anti-σ factor CseE. Microbiology 154:915–923
Park JS, Shin S, Kim ES, Kim P, Kim Y, Lee HS (2011) Identification of SpiA that interacts with Corynebacterium glutamicum WhcA using a two‐hybrid system. FEMS Microbiol Lett 322:8–14
Park JS, Lee JY, Kim HJ, Kim ES, Kim P, Kim Y, Lee HS (2012) The role of Corynebacterium glutamicum spiA gene in whcA-mediated oxidative stress gene regulation. FEMS Microbiol Lett 331:63–69
Pesakhov S, Benisty R, Sikron N, Cohen Z, Gomelsky P, Khozin-Goldberg I, Dagan R, Porat N (2007) Effect of hydrogen peroxide production and the Fenton reaction on membrane composition of Streptococcus pneumoniae. Biochim Biophys Acta (BBA) Biomembr 1768:590–597
Pfeifer-Sancar K, Mentz A, Rückert C, Kalinowski J (2013) Comprehensive analysis of the Corynebacterium glutamicum transcriptome using an improved RNAseq technique. BMC Genomics 14:888
Rocha-Martin J, Vega D, Bolivar JM, Godoy CA, Hidalgo A, Berenguer J, Guisan JM, Lopez-Gallego F (2011) New biotechnological perspectives of a NADH oxidase variant from Thermus thermophilus HB27 as NAD+-recycling enzyme. BMC Biotechnol 11:101
Rybniker J, Nowag A, Van Gumpel E, Nissen N, Robinson N, Plum G, Hartmann P (2010) Insights into the function of the WhiB‐like protein of mycobacteriophage TM4—a transcriptional inhibitor of WhiB2. Mol Microbiol 77:642–657
Sambrook J, Russell DW, Russell DW (2001) Molecular cloning: a laboratory manual. Cold spring harbor laboratory press Cold Spring. Harbor, New York
Sauvageot N, Ladjouzi R, Benachour A, Rince A, Deutscher J, Hartke A (2012) Aerobic glycerol dissimilation via the Enterococcus faecalis DhaK pathway depends on NADH oxidase and a phosphotransfer reaction from PEP to DhaK via EIIADha. Microbiology 158:2661–2666
Schäfer A, Tauch A, Jäger W, Kalinowski J, Thierbach G, Pühler A (1994) Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 145:69–73
Singh R, Wiseman B, Deemagarn T, Donald LJ, Duckworth HW, Carpena X, Fita I, Loewen PC (2004) Catalase-peroxidases (KatG) exhibit NADH oxidase activity. J Biol Chem 279:43098–43106
Singh A, Guidry L, Narasimhulu KV, Mai D, Trombley J, Redding KE, Giles GI, Lancaster JR Jr, Steyn AJ (2007) Mycobacterium tuberculosis WhiB3 responds to O2 and nitric oxide via its [4Fe-4S] cluster and is essential for nutrient starvation survival. Proc Natl Acad Sci U S A 104:11562–11567
Singh A, Crossman DK, Mai D, Guidry L, Voskuil MI, Renfrow MB, Steyn AJ (2009) Mycobacterium tuberculosis WhiB3 maintains redox homeostasis by regulating virulence lipid anabolism to modulate macrophage response. PLoS Pathog 5:e1000545
Smith LJ, Stapleton MR, Fullstone GJ, Crack JC, Thomson AJ, Le Brun NE, Hunt DM, Harvey E, Adinolfi S, Buxton RS (2010) Mycobacterium tuberculosis WhiB1 is an essential DNA-binding protein with a nitric oxide-sensitive iron-sulfur cluster. Biochem J 432:417–427
von der Osten CH, Gioannetti C, Sinskey AJ (1989) Design of a defined medium for growth of Corynebacterium glutamicum in which citrate facilitates iron uptake. Biotechnol Lett 11:11–16
Yamamoto Y, Pargade V, Lamberet G, Gaudu P, Thomas F, Texereau J, Gruss A, Trieu‐Cuot P, Poyart C (2006) The Group B Streptococcus NADH oxidase Nox‐2 is involved in fatty acid biosynthesis during aerobic growth and contributes to virulence. Mol Microbiol 62:772–785
Yang X, Ma K (2007) Characterization of an exceedingly active NADH oxidase from the anaerobic hyperthermophilic bacterium Thermotoga maritima. J Bacteriol 189:3312–3317
Yoshihama M, Higashiro K, Rao EA, Akedo M, Shanabruch WG, Follettie MT, Walker GC, Sinskey AJ (1985) Cloning vector system for Corynebacterium glutamicum. J Bacteriol 162:591–597
Zarepour M, Kaspari K, Stagge S, Rethmeier R, Mendel RR, Bittner F (2010) Xanthine dehydrogenase AtXDH1 from Arabidopsis thaliana is a potent producer of superoxide anions via its NADH oxidase activity. Plant Mol Biol 72:301–310
Zhang GC, Liu JJ, Ding WT (2012) Decreased xylitol formation during xylose fermentation in Saccharomyces cerevisiae due to overexpression of water-forming NADH oxidase. Appl Environ Microbiol 78:1081–1086
Zhang X, Zhang R, Bao T, Rao Z, Yang T, Xu M, Xu Z, Li H, Yang S (2014) The rebalanced pathway significantly enhances acetoin production by disruption of acetoin reductase gene and moderate-expression of a new water-forming NADH oxidase in Bacillus subtilis. Metab Eng 23:34–41
Zheng F, Long Q, Xie J (2012) The function and regulatory network of WhiB and WhiB-like protein from comparative genomics and systems biology perspectives. Cell Biochem Biophys 63:103–108
Acknowledgments
This work was supported by a Korea University Grant to H.-S. Lee.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Park, J.C., Kim, Y. & Lee, HS. Involvement of the NADH oxidase-encoding noxA gene in oxidative stress responses in Corynebacterium glutamicum . Appl Microbiol Biotechnol 99, 1363–1374 (2015). https://doi.org/10.1007/s00253-014-6327-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-6327-x