Abstract
As their environments change, microbes experience various threats and stressors, and in the hypercompetitive microbial world, dynamism and the ability to rapidly respond to such changes allow microbes to outcompete their nutrient-seeking neighbors. Viewed in that light, the very difference between microbial life and death depends on effective stress response mechanisms. In addition to the more commonly studied temperature, nutritional, and chemical stressors, research has begun to characterize microbial responses to physical stress, namely low-shear stress. In fact, microbial responses to low-shear modeled microgravity (LSMMG), which emulates the microgravity experienced in space, have been studied quite widely in both prokaryotes and eukaryotes. Interestingly, LSMMG-induced changes in the virulence potential of several Gram-negative enteric bacteria, e.g., an increased enterotoxigenic Escherichia coli-mediated fluid secretion in ligated ileal loops of mice, an increased adherent invasive E. coli-mediated infectivity of Caco-2 cells, an increased Salmonella typhimurium-mediated invasion of both epithelial and macrophage cells, and S. typhimurium hypervirulence phenotype in BALB/c mice when infected by the intraperitoneal route. Although these were some examples where virulence of the bacteria was increased, there are instances where organisms became less virulent under LSMMG, e.g., hypovirulence of Yersinia pestis in cell culture infections and hypovirulence of methicillin-resistant Staphylococcus aureus, Enterococcus faecalis, and Listeria monocytogenes in a Caenorhabditis elegans infection model. In general, a number of LSMMG-exposed bacteria (but not all) seemed better equipped to handle subsequent stressors such as osmotic shock, acid shock, heat shock, and exposure to chemotherapeutics. This mini-review primarily discusses both LSMMG-induced as well as bona fide spaceflight-specific alterations in bacterial virulence potential, demonstrating that pathogens’ responses to low-shear forces vary dramatically. Ultimately, a careful characterization of numerous bacterial pathogens’ responses to low-shear forces is necessary to evaluate a more complete picture of how this physical stress impacts bacterial virulence since a “one-size-fits-all” response is clearly not the case.
Similar content being viewed by others
Introduction
For over the past 30 years during the existence of the National Aeronautics and Space Administration (NASA) Space Shuttle Program, scientists and astronauts alike have been concerned with the colonization of microbes (including molds, bacteria, and viruses) in Space Shuttles and, later, the International Space Station (ISS). These concerns are legitimate as such pathogens can spread from one astronaut to another while in flight, particularly if astronauts are immunocompromised (Stowe et al. 2011; Maule et al. 2009; Vesper et al. 2008; La Duc et al. 2004). From a microbe’s perspective, an astronaut, like any other human, is one large biome comprised of eukaryotic cells (including all immunologically relevant cells), bacterial normal flora/microbiota, and the virome which inhabits both eukaryotic cells and normal bacterial flora. Ultimately, human health is influenced by the association of all of the aforementioned components of the human biome. Any perturbations in immune function can lead to a disease state, and this issue is heightened in space where a compromised immune system may encounter hypervirulent pathogens.
Unfortunately, several members of the human normal flora/microbiota are notable opportunistic pathogens, and within an immunocompromised host, they pose an increased risk of morbidity. Furthermore, spaceflight (and its various associated conditions, e.g., microgravity and ionizing space radiation/solar flares) has been demonstrated to dysregulate the immune system (IS) of astronauts via the skewing of their cytokine profiles to a T helper cell 2 (Th2) bias. More specifically, T cells that characterized post-spaceflight were found to be less robust in their production of both interleukin-2 (IL-2) and interferon gamma (IFN-γ) (Crucian et al. 2008). A Th2-biased immune response could potentially leave an astronaut prone to intracellular pathogen-mediated infections, including viral insults. It came as no surprise then that immunocompromised astronauts on both short- and long-term space missions to the ISS (11 and 180 days, respectively) demonstrated elevated Epstein Barr Virus (EBV) transcripts, indicating that a compromised IS failed to control latent EBV infections (Stowe et al. 2011).
In addition to the adaptive arm of the IS, the innate arm was also negatively impacted by spaceflight. More specifically, neutrophils were found to have reduced phagocytic and oxidative burst potential (Kaur et al. 2004). Having both arms of the compromised IS could prove particularly dangerous to the future prolonged human exploration of space where limited medications and supplies could prove life-threatening in the event of microbial infections. This becomes even more complicated by the fact that many such pathogens are highly communicable and, in space, can easily spread by direct contact, respiratory droplets, and saliva. Furthermore, in a study evaluating the peripheral blood of astronauts who returned to earth following a space mission, their monocytic responses to the endotoxin lipopolysaccharide (LPS) at various time points revealed altered cytokine profiles. More specifically, IL-6 and IL-8 (both involved in inflammation and fever) production was lower than in control sample subjects (Kaur et al. 2008).
Studies have also emerged demonstrating that bacteria grown under low-shear modeled microgravity (LSMMG) or in space exhibited increased resistance to antimicrobial chemotherapeutics (Juergensmeyer et al. 1999; Nickerson et al. 2003; Nickerson et al. 2004; Lynch et al. 2006; Klaus and Howard 2006). Coupled with an immunologically compromised host, a bacterial pathogen equipped with such enhanced resistance represents a danger to the flight crew on space missions. In other words, in space, an immunocompromised host is more prone to becoming infected with a bacterial pathogen potentially resulting in lethal consequences. Alternatively, immunocompetent individuals could become infected by opportunistic pathogens that have become hypervirulent in space. This mini-review focuses on bacterial responses to space and space-like stress as they relate to microbial virulence potential, as well as cross-reference some non-pathogenic bacterial surrogate strains to provide an overview on LSMMG and its effects on host susceptibility to microbial infections.
Spaceflight experiments and their inherent limitations
Overview of spaceflight experiments
There is a storied history of studying over 100 microbial cultures in space, many of which were bacterial pathogens (cited in Tixador et al. 1985; Juergensmeyer et al. 1999; McLean et al. 2001; Nickerson et al. 2000; Nickerson et al. 2004; Wilson et al. 2007; Wilson et al. 2008; Crabbé et al. 2011). Some of the earliest spaceflight microbial studies characterized growth rates and biomass. These studies revealed that bacterial cultures grown in space tended to grow more densely, mostly due to decreased lag phase coupled with a prolonged log phase (Nickerson et al. 2004). As a result, NASA initiated studies to rapidly identify potential pathogens and their resistance to antimicrobial agents during space missions to protect the health of astronauts. In earlier experiments, minimum inhibitory concentrations (MICs) of antibiotics were evaluated in Staphylococcus aureus and Escherichia coli. Bacteria appeared to exhibit enhanced antimicrobial resistance relative to ground controls (Tixador et al. 1985). Those results were not recapitulated when MIC studies were carried out on solid media, strongly suggesting that earlier observation of enhanced resistance might be specific to growth in liquid culture in which low-shear forces were experienced by the microbe (Kacena and Todd 1999).
Regardless when bacteria cause an infection and disseminate within a host, they often encounter bodily fluids (e.g., blood when bacteremic), experiencing low-shear force conditions (Norman et al. 2008), and encountered in space microgravity regardless of whether that infected individual is an astronaut in spaceflight or a human on earth. Also, it has been suggested that mechanotransductive signaling, which converts mechanical stimuli into chemical/physiological activity, might occur on bacterial surfaces promoting physiological responses to that environment as a protective measure (Ingber 1999). In fact, mechanical shear forces have been demonstrated as having an impact on bacterial physiology and pathogenesis in several studies (Hamill and Martinac 2001; Thomas et al. 2002; Nickerson et al. 2003)
To that end, there still exists the need to more rapidly and more accurately determine bacterial identifications, concentrations, and their susceptibility to countermeasures/antimicrobials in space and in other low-shear environments with high reproducibility. In addressing that need directly, a card-based assay to rapidly detect MICs of antimicrobials to various pathogenic microbes while flown in space was developed that relies on a simple colorimetric readout that the crew can easily interpret (Jorgensen et al. 1997). Unfortunately, sensitivity issues and the need to isolate pure cultures limit this tool’s use. Near real-time detection and identification of bacterial pathogens (as well as determination of their antimicrobial sensitivities) are highly relevant since bacteria (e.g., Burkholderia spp. and Bacillus spp.) have been readily isolated from the ISS and its water supply in the past (Castro et al. 2004; La Duc et al. 2004; Klaus and Howard 2006). In efforts of preventing bacterial infections during space missions, a highly sensitive biosensor that could detect trace amounts of microbial products (e.g., LPS, techoic acid, or DNA sequence) on the ISS (or a space shuttle) and report back their presence or absence in real time would prove extremely useful. While such a tool could identify bacterial presence, it would not be able to address the MIC alteration problem discussed above.
Despite the studies described above, virulence studies on space-flown pathogens have been rather limited in scope. This is due to a number of reasons, namely limited spaceflight missions, limited physical space on those missions, potential risk to flight crew, and limited funding to conduct such experiments. Despite the aforementioned limitations, several seminal studies have been carried out and are discussed in this review.
Salmonellae spaceflight studies
Onboard the STS-115 Space Shuttle mission, Salmonella typhimurium was grown in both nutrient-rich Lennox broth (LB) (Wilson et al. 2007) and M9 (minimal salts) medium (Wilson et al. 2008). Following space growth in LB medium, S. typhimurium exhibited 167 differentially expressed genes in addition to 73 differentially produced proteins (as observed through tandem mass spectrometry coupled to liquid chromatography). Further, space-flown S. typhimurium displayed a hypervirulent phenotype (in an orogastric BALB/c model of infection) relative to the ground control culture as evidenced by a ∼3-fold decrease in LD50, increased percent mortality (at various infection doses), and decreased time to death (at a 107 infectious dose). In one representative experiment, 30 % of spaceflight-grown S. typhimurium-infected mice had died compared to 0 % death in normal gravity-grown S. typhimurium-infected mice 7 days post-infection (Wilson et al. 2007). Interestingly, of the 69 and 98 up- and downregulated genes, respectively, many were found to be part of the Hfq regulon (Wilson et al. 2007). Following spaceflight, a Pseudomonas aeruginosa gene expression study revealed that hfq was downregulated. Hfq, encoded by the hfq gene, is a small RNA binding protein that globally regulates gene expression. In general, transcripts under positive control by Hfq were found to be downregulated while transcripts under negative control by Hfq were upregulated (Crabbé et al. 2011). Furthermore, it appeared that Hfq could potentially be a broad global regulator of spaceflight-responsive genes at least in related Gram-negative bacteria. These data strongly suggest that Hfq is a space-responsive gene product necessary for rapid bacterial reprogramming in a low-shear environment. This could help shed light on the mechanism by which Hfq, identified as a virulence-associated gene product in numerous pathogens including Yersinia spp., Salmonella spp., and Vibrio spp. (Chao and Vogel 2010), influences bacterial pathogenesis. Further investigation could potentially uncover novel therapeutic targets.
In addition to the study described above where S. typhimurium was grown during the STS-115 mission in LB and minimal M9 media (Wilson et al. 2007), the organism was grown in space for the second time on board the STS-123 Space Shuttle mission, in LB medium, minimal M9 medium, and LB medium supplemented with five salts contained in M9 medium. Murine infections and gene expression profile studies were carried out following growth in the aforementioned conditions and returned to earth, revealing that flagellar genes involved in motility were downregulated (Wilson et al. 2008). Interestingly, only LB (nutrient-rich)-grown S. typhimurium exhibited hypervirulence in a murine model of infection, resulting in earlier time to death and decreased LD50 rates, following return to earth. In sharp contrast, neither growth in the minimal M9 medium nor in the LB medium spiked with five M9 salts promoted S. typhimurium hypervirulence. More specifically, spaceflight-grown S. typhimurium in M9 and LB with five M9 salts exhibited slightly increased LD50s as well as similarly delayed time to death in murine infections relative to ground controls respectively (Wilson et al. 2008). This demonstrated that nutritional composition of growth medium influences S. typhimurium virulence, as seen in murine models of infection.
P. aeruginosa spaceflight studies
An opportunistic pathogen, P. aeruginosa (a major cause of nosocomial infections in burn patients), was flown onboard the STS-95 Space Shuttle mission in 2001 to determine whether biofilm was produced in space. This is a worthy question since biofilm itself is a virulence-associated factor of many bacterial pathogens. Interestingly, P. aeruginosa was found to produce biofilm in space (McLean et al. 2001). More recently, P. aeruginosa was flown on two separate Space Shuttle Atlantis missions (STS-132 and STS-135), and again, increased biofilm production was observed relative to the ground control cultures. Furthermore, space microgravity produced thicker biofilms that exhibited motility-dependent, aberrant architectures (column and canopy structure) relative to the ground control cultures (Kim et al. 2013a). Interestingly, when phosphate and oxygen levels were reduced, P. aeruginosa exhibited increased final biomass/enhanced growth during spaceflight (Kim et al. 2013b). Whereas the contents of M9 salts diminished salmonellae hypervirulence in murine infections following spaceflight and phosphate was speculated to be the key factor (Wilson et al. 2008), phosphate also positively influenced P. aeruginosa growth during spaceflight (Kim et al. 2013b). It seems that some spaceflight responses of various bacteria seem dependent on specific nutritional factors (e.g., the presence of phosphate).
Seminal spaceflight infection study
In a virulence study by Hammond et al. (2013), three bona fide bacterial pathogens, Listeria monocytogenes, methicillin-resistant S. aureus (MRSA), and Enterococcus faecalis, were exposed to spaceflight (actual microgravity) or LSMMG (generated using a clinostat) and compared to ground normal gravity (NG) controls in their ability to kill larval and adult wild-type Caenorhabditis elegans. Nematodes and pathogens were mixed and then co-cultured for 48 h, during which, bacterial virulence was measured by evaluating nematode mortality. All three pathogens demonstrated attenuated virulence (as measured by C. elegans mortality) in spaceflight. Moreover, this was the first experiment that assayed virulence of bacterial pathogens in space (true microgravity environment). Interestingly, when the group characterized the LSMMG effect, the decrease in the virulence of all three pathogens was less pronounced, but the general trend of no observed hypervirulence was maintained (Hammond et al. 2013). Despite this study advancing the field of space microbiology by employing a spaceflight (true microgravity) infection system, most virulence studies employing C. elegans involve excessive biofilm production resulting in starvation and/or GI infections (Balla and Troemel 2013). Therefore, mortality measured (in the nematode) may not be a consequence of virulence specifically being altered rather of modulated biofilm formation (an indirect virulence-associated factor). To expand the findings, ground-based analogs (e.g., High Aspect Ratio Vessels [HARVs]) could be employed to produce LSMMG culturing conditions for the aforementioned three pathogens prior to murine infections (or other model infection systems). However, employing additional infection model systems in space would be too technically challenging, so currently, the unique C. elegans infection system is best suited for space infection studies. Unfortunately, spaceflight experiments involving the salmonellae, pseudomonads, or other bacterial pathogens have been limited due to the reasons described above; this resulted in a need to develop ground-based systems that emulated the microgravity conditions experienced in space.
The need for inexpensive ground-based alternatives to spaceflight
As described above, the obvious limitations in replicating spaceflight experiments have led to the need for less expensive ground-based alternatives to characterize the responses to space-like stress, including microgravity. This has become even more imperative since the retirement of NASA’s Space Shuttle fleet. Below, a more detailed discussion about the various ground-based LSMMG systems is provided.
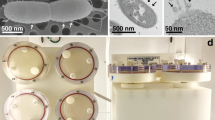
To create LSMMG, several systems have been developed and employed for the purpose of eukaryotic and prokaryotic cell cultivation and study (Nickerson et al. 2004; Chopra et al. 2006; Rosenzweig et al. 2010; Lawal et al. 2010). These systems include clinostats (used for over a century), random positioning machines, magnetic levitation techniques (relatively recent development that employs varied magnetic fields), and the more commonly used rotating wall vessels, including the HARV that was developed by NASA scientists David Wolf (astronaut, electrical engineer, and a physician), Tinh Trinh (mechanical engineer), and Ray Schwarz (Chief Engineer) (http://www.nasa.gov/offices/oct/home/feature_hof_prt.htm). HARVs allow for the facile emulation of microgravity by simply rotating the culture on an axis perpendicular to the force of gravity; doing so offsets the weight of the cells by low-shear fluid forces which suspend the bacteria in a state of constant free fall. To apply normal gravity forces, the HARV is rearranged such that the axis of rotation is parallel to the force of gravity (Fig. 1). After being granted a license from NASA, Ray Schwarz (Chief Engineer) and Charles D. Anderson (Project Manager) made HARVs commercially available as co-founders of Synthecon Inc. (Houston, TX). These HARVs are the most widely used system in the literature for the characterization of microbes grown under LSMMG (Nickerson et al. 2004; Chopra et al. 2006; Lawal et al. 2010).
High aspect ratio vessel (HARV) orientations. a Picture of the HARV bioreactor. b When the axis of rotation is perpendicular to the force of gravity, cells within the HARV experience LSMMG. However, when the axis of rotation is arranged in a manner parallel to the force of gravity, cells within the HARV experience normal gravity (NG)
Terrestrial LSMMG studies of E. coli and the salmonellae
In agreement with the earlier finding that spaceflight resulted in increased biofilm production in P. aeruginosa (McLean et al. 2001), E. coli also demonstrated enhanced biofilm formation following LSMMG culture. This phenotype of LSMMG-cultured E. coli enabled better resistance to antibiotics, altered pH, osmolarity, and oxidative stresses (Lynch et al. 2006). Furthermore, LSMMG-enhanced resistance to acid and osmolarity stress was dependent on sigma factor σs, a general stress response factor (encoded by the rpoS gene), during the stationary phase when its concentration was 30 % higher than in exponential growth phase (Lynch et al. 2004). However, when the E. coli MG 1655 transcriptome was evaluated following LSMMG exposure, no specific LSMMG-responsive genes were identified (Tucker et al. 2007). The observation made by Tucker et al. (2007) that cell membrane-associated genes were upregulated coupled with the earlier finding that LSMMG enhances biofilm formation (Lynch et al. 2006) suggests that LSMMG exerts its effect on the cell surface, possibly by way of some general stress response factor like σs (Lynch et al. 2004). Further, a recent study reported that none of the trp operon genes (involved in the production of tryptophan) were involved in either the enhanced acid stress tolerance of E. coli or its decreased oxidative stress tolerance following LSMMG growth (Soni et al. 2014). Neither was the trp operon genes found to play a role in S. typhimurium’s increased sensitivity to both acid and oxidative stress following LSMMG growth (Soni et al. 2014). Collectively, these data suggest that tryptophan metabolism does not play a key role during LSMMG exposure in two notable Gram-negative enteric bacteria; however, the global stress response factor, σs, was required for enhanced resistance to acid and osmolarity stress (Lynch et al. 2004).
S. typhimurium has also been subjected to numerous ground-based LSMMG studies that employed the HARV in addition to the organism’s two spaceflight experiments, as discussed above (Wilson et al. 2007, 2008). Although the hypervirulence phenotype observed in the LSMMG-cultured salmonellae resembled those of their space-grown counterparts (Wilson et al. 2002a, b, 2007, 2008), differences were observed in the differentially expressed genes. Whereas in one study LSMMG induced differential gene expression of 163 genes including the downregulation of ten type III secretion system (T3SS) virulence genes (Wilson et al. 2002b), a second independent study using stringent statistical analysis revealed only 22 genes differentially expressed (including the upregulation of the virK virulence gene involved in resistance to host antimicrobial peptides [Spencer et al. 2010]) following LSMMG exposure (Chopra et al. 2006). Interestingly, while spaceflight induced differential expression of 167 genes in the salmonellae (Wilson et al. 2007), a number similar to those differentially expressed in one LSMMG exposure experiment (Wilson et al. 2002b), there were qualitative differences in the types of genes differentially expressed. For example, under LSMMG conditions, a (T3SS) virulence gene sipD was downregulated more than 2-fold (Wilson et al. 2002b) whereas following spaceflight sipC (a virulence gene involved in cell invasion) was upregulated 6.27-fold (Wilson et al. 2007). These differences underscore the fact that although LSMMG might closely emulate spaceflight, it does not truly mimic a spaceflight. As alluded earlier, LSMMG has been found to induce salmonellae hypervirulence in murine models of infection. More specifically, mice challenged with LSMMG-cultured salmonellae demonstrated increased mortality 6 days post-infection (p.i.) relative to mice infected with NG-grown salmonellae. In one experiment, at 10 days p.i., only 20 % of mice challenged with LSMMG-cultured salmonellae survived, as compared to 60 % of those infected with NG control salmonellae (Nickerson et al. 2000). In another set of independent experiments, mice were tail-suspended to emulate microgravity experienced by the host and, when challenged with LSMMG-grown salmonellae, experienced 100 % mortality 3 days p.i., as compared to 60 % mortality when infected with NG-grown salmonellae at the same time point (Chopra et al. 2006). These data strongly agree with the hypervirulence observed in space-flown salmonellae that exhibited faster time to death in murine infections and decreased LD50, relative to mice infected with ground control salmonellae (Wilson et al. 2007, 2008).
Surprisingly, the enhanced virulence potential of S. typhimurium was not due to increased expression of virulence genes that encode the two independent T3SSs located on the Salmonella pathogenicity islands 1 and 2 (Wilson et al. 2002b). The two aforementioned T3SSs are required for both invasion and survival within eukaryotic host cells respectively. Such a revelation suggests that LSMMG and by extension spaceflight could be enhancing the Salmonella virulence potential (Wilson et al. 2002a, 2008; Chopra et al. 2006) through a T3SS-independent manner. Through which specific mechanism(s), LSMMG enhances the S. typhimurium virulence potential remains unclear; however, it could be due to increased biofilm formation that occurs under LSMMG and spaceflight (Lynch et al. 2006; Wilson et al. 2007). Alternatively, increased expression of the virK gene in S. typhimurium under LSMMG condition could lead to increased bacterial virulence as VirK contributes to remodeling of the bacterial outer membrane in response to the host environment (Chopra et al. 2006). Perhaps, is it the observed increased resistance of LSMMG-grown salmonellae to oxidative stress (Pacello et al. 2012) that promotes enhanced virulence potential? In that study, enhanced oxidative stress resistance was supported by the observation that KatG and KatY (catalase enzymes) were upregulated in the LSMMG-grown salmonellae in a Hfq-, RpoE-, RpoS-, and OxyR- (global regulators) independent manner (Pacello et al. 2012). However, another study reported that, following LSMMG growth, S. typhimurium experienced increased sensitivity to oxidative stress and that the increased sensitivity was RpoS-independent (Wilson et al. 2002a). Considering all of the abovementioned data, the precise mechanism by which S. typhimurium experiences hypervirulence following LSMMG growth in nutrient-rich medium remains unclear.
To determine specifically if any of the M9 salts could impact the previously observed S. typhimurium-increased acid tolerance in LB medium following LSMMG exposure (Nickerson et al. 2000), Wilson et al. (2008) demonstrated that it was the exact M9 salt components containing phosphate (specifically Na2HPO4 and KH2PO4) that counteracted the salmonellae-increased acid tolerance observed following LSMMG growth in LB medium (Wilson et al. 2008). More specifically, the addition of the aforementioned salts to LB medium resulted in roughly no S. typhimurium-increased acid resistance (at pH 3.5) as compared to the NG-grown control. In sharp contrast, when S. typhimurium was grown in LB medium under LSMMG condition, there was an observed ∼6.5-fold increased acid resistance relative to the NG-grown control. Taken together, environmental/nutritional conditions (e.g., the presence of nutritional factors like phosphate ions) can reprogram bacteria in space and during LSMMG growth resulting in diminished hypervirulence and increased acid tolerance respectively (Wilson et al. 2008).
Taken together, these data suggest that for virulence studies, LSMMG is an appropriate model system for the study of low-shear forces associated with microgravity (Rosenzweig et al. 2010). However, considering the differences in gene expression profiles between LSMMG- and spaceflight-exposed salmonellae, it is important to stress that although LSMMG is the closest one can get to a spaceflight study, LSMMG is not spaceflight.
Terrestrial LSMMG studies of pathogenic E. coli strains, P. aeruginosa, Yersinia pestis, and other Enterobacteriaceae family members
In addition to LSMMG studies involving the hypervirulent salmonellae (discussed in detail above), other bacterial pathogens have also been characterized following LSMMG growth, including enterotoxigenic E. coli (ETEC) (Chopra et al. 2006), enteropathogenic E. coli (EPEC) (Chopra et al. 2006), adherent-invasive E. coli (AIEC) (Allen et al. 2008), enterohemorrhagic E. coli (EHEC) (Kim et al. 2014), P. aeruginosa (Crabbé et al. 2008, 2010), and Y. pestis (Lawal et al. 2010, 2013). The general trend for most Gram-negative pathogens (with the exception of Y. pestis) following LSMMG growth was enhanced virulence potential (Table 1). In the case of P. aeruginosa, there was enhanced production of biofilm which facilitates bacterial adherence to surfaces and is thus considered a virulence-associated factor (Crabbé et al. 2008). With regard to ETEC, LSMMG enhanced its virulence potential through a significant upregulation of the heat labile (LT-1) enterotoxin which caused increased fluid secretory responses in the ligated ileal loops of mice (Chopra et al. 2006), whereas LSMMG-grown AIEC became hyperadherent when co-cultured with a gastrointestinal epithelial cell line (Allen et al. 2008). In the case of EHEC, increased biomass (as a result of increased cell size and numbers) was observed following LSMMG growth in both nutrient-rich and nutrient-deprived media (Kim et al. 2014).
To date, several exceptions to the above discussed trend of LSMMG-grown Gram-negative hypervirulent pathogens have emerged. In one such example, LSMMG-grown Y. pestis exhibited a reduced capacity to proliferate in cultured macrophage-like cells (murine RAW 264.7) and was impaired in its ability to induce HeLa cell rounding and cytotoxicity. Further study revealed that this decreased virulence potential resulted from reduced expression and subsequent secretion of T3SS effector proteins necessary for optimal virulence (Lawal et al. 2010). However, following LSMMG growth, Y. pestis did not exhibit alterations in growth kinetics, cold resistance, or antibiotic sensitivity (Lawal et al. 2013). Further, LSMMG growth did not significantly influence Y. pestis virulence as observed in a Swiss-Webster intraperitoneal (IP) murine infection model (Lawal et al. 2013) in agreement with what was previously observed in a RAW 264.7 cell culture infection model (Lawal et al. 2010).
Since LSMMG appeared to repress Y. pestis’ T3SS expression and function, a Y. pestis ΔymoA deletion mutant, deficient in the YmoA DNA histone-like protein involved in regulating gene expression (which suppresses T3SS expression), was grown under LSMMG. Surprisingly, similar to what was observed in the Y. pestis wild-type strain, LSMMG also reduced T3SS expression and function in the T3SS-dysregulated isogenic ΔymoA mutant (Lawal et al. 2013). These data suggested that LSMMG was specifically regulating (negatively) the Y. pestis virulence plasmid pCD1 (which encodes its T3SS) and that this negative regulation occurs even when the T3SS is dysregulated (as in the absence of YmoA). Strikingly, the ΔymoA mutant was significantly more virulent in a murine model of infection than the isogenic wild-type strain following growth under LSMMG conditions, despite inducing less cytotoxicity in a cell culture infection model. Taken together, LSMMG did enhance the in vivo virulence potential of a Y. pestis ΔymoA mutant (relative to its isogenic wild-type strain) through a T3SS-independent unknown mechanism.
More recently, following LSMMG growth, three notable enteric opportunistic pathogens (capable of causing a wide array of nosocomial infections) were found to respond quite differently. Whereas LSMMG promoted enhanced acid stress resistance in Serratia marcescens, no enhanced resistance and even diminished acid resistance was observed in LSMMG-grown Citrobacter freundii and Enterobacter cloacae, respectively (Soni et al. 2014). Likewise, the oxidative stress resistance of C. freundii and E. cloacae was similarly diminished (Soni et al. 2014). These data further underscore the fact that not all bacterial pathogens respond similarly to LSMMG.
Terrestrial LSMMG studies of S. aureus, L. monocytogenes, and E. faecalis
Interestingly, when S. aureus was exposed to LSMMG, following growth in a rotating wall vessel, it also exhibited downregulated expression of hfq (Castro et al. 2011) in a manner consistent with what was formerly observed in P. aeruginosa (Crabbé et al. 2011) and S. typhimurium (Wilson et al. 2007). However, unlike the salmonellae which exhibited hypervirulence phenotype in the murine model of infection (Nickerson et al. 2000; Chopra et al. 2006), the S. aureus response to LSMMG resembled that of the yersiniae (Lawal et al. 2010), resulting in diminished virulence potential, as observed by increased sensitivity to oxidative stress (Castro et al. 2011). In an asynchronous ground-based LSMMG study (coupled to the spaceflight study), clinorotation of MRSA did not result in alterations of virulence in a C. elegans infection model system measuring nematode cytotoxicity (Hammond et al. 2013). In the same report, clinorotation was also used to co-culture L. monocytogenes or E. faecalis with C. elegans larval or adult nematodes. Whereas E. faecalis exhibited hypovirulence when infecting larval C. elegans (but not adults), L. monocytogenes experienced no significant change in its virulence using the same infection model system (Hammond et al. 2013).
The fact that Y. pestis, S. aureus, MRSA, E. faecalis, and L. monocytogenes have responded to LSMMG (hypovirulence) differently than ETEC, EPEC, AIEC, S. typhimurium, and P. aeruginosa responded to LSMMG (hypervirulence or an increased virulence potential, as in the case of P. aeruginosa) highlights the fact that closely related bacteria have adopted very diverse pathogenic and stress response strategies over time. These differences underscore the need to broaden our knowledge of how additional bacterial pathogens respond to LSMMG.
Applying spaceflight and LSMMG knowledge to the ground
Our ever-expanding knowledge of the effects of LSMMG on bacterial phenotypes and virulence potentials has much wider implications than exclusively improving prolonged manned space exploration and ensuring the health of our astronauts. Another goal would be to apply our understanding of the effects of LSMMG to ground-based microbiological processes. From a commercial perspective, growth of microbes under LSMMG could be exploited to enhance the production of certain desired microbiological products. However, some products/metabolites have been inhibited following bacterial LSMMG growth. When evaluating antibiotic production of beta lactams (made by Streptomyces clavuligerus) (Fang et al. 1997a), gramicidin S (made by Bacillus brevis) (Fang et al. 1997b), rapamycin (made by Streptomyces hygroscopicus) (Fang et al. 2000), and microcin B17 (made by E. coli) (Fang et al. 1997c) following LSMMG growth, production of all the aforementioned antibiotics was inhibited with the exception of gramicidin S (Demain and Fang 2001).
In addition to commercial aspects, there is fundamental interest in how microbes evolved on this planet, and without LSMMG and the more costly spaceflight alternative, we cannot evaluate growth or phenotypes of microbes in the absence of one of the biggest evolutionary influences on life today as we know it, namely gravitational force. In this vein, a study characterizing the effects of LSMMG growth on haloarchaea bacteria was carried out. Archaea are the oldest life forms on our planet and thus represent an interesting model organism to address the evolutionary question posed above using LSMMG methodologies (Dornmayr-Pfaffenhuemer et al. 2011).
However, one of the most relevant and meaningful applications of LSMMG studies to terrestrial life on earth is to supplement our understanding of the various virulence mechanisms of pathogenic bacteria. In the case of Y. pestis and P. aeruginosa, both of which can cause pneumonic infections (pneumonic plague and chronic lung infections in cystic fibrosis patients, respectively), it becomes important to understand how the pathogen responds to the various shear forces it experiences during the infection process. Viewed in that light, one can envision how shear forces in blood would be quite different than those found in the lung’s mucosa. Further, bacteria could experience low-shear forces when internalized within a macrophage phagosome during an immune cell encounter. In some cases, pathogens cause fulminant infections, prompting us to consider low-shear force kinetics during bacterial infections. Such an understanding could influence clinical decisions regarding a patient’s treatment program.
Conclusion
By no means should efforts to characterize bacterial responses to LSMMG and spaceflight be limited to pathogens relevant to spaceflight. As described above, a detailed and more exhaustive list of pathogens should be evaluated following growth in LSMMG to broaden our understanding of bacterial responses to shear forces that are experienced during infection, colonization, and dissemination in the human host. Currently, too few pathogens have been evaluated under LSMMG and that list needs to be greatly expanded. In fact, LSMMG studies should be expanded to include notable Gram-positive pathogens [beyond the S. aureus (Castro et al. 2011), E. faecalis, MRSA, and L. monocytogenes (Hammond et al. 2013)]. Further, additional human viruses capable of causing latent infections and even additional fungal pathogens should be included in future studies.
The yeast, Candida albicans, an opportunistic pathogen, was evaluated following LSMMG growth and revealed increased filamentation, enhanced biofilm formation, and antimicrobial resistance (Altenburg et al. 2008; Searless et al. 2011). More recently, C. albicans was characterized following spaceflight and enhanced random budding and 8.3 % differentially expressed genes were observed (including increased expression of genes involved in aggregation and oxidative stress responses) (Crabbé et al. 2013). In a separate spaceflight experiment, C. albicans was found to induce less mortality in a larval and adult C. elegans model of infection (Hammond et al. 2013). In addition, the non-pathogenic yeast model organism, Saccharomyces cerevisiae, was found to overexpress genes associated with budding, resulting in random budding along the cell surface (Purevdorj-Gage et al. 2006). In a separate study, LSMMG was found to induce differential expression of 1,372 genes in S. cerevisiae, possibly providing insights into how other pathogenic fungi might respond to LSMMG (Sheehan et al. 2007). However, additional studies examining the effects of LSMMG on fungi should be carried out to establish a more comprehensive list of microbial responses to LSMMG. Such a list will prove valuable not only for astronauts on prolonged spaceflight missions where medications are limited and access to health-care facilities is impossible but also to the infirmed on earth.
Ultimately, a careful evaluation of the space microbiology literature reveals one underlying truth. There exists no common, predictable bacterial response to LSMMG and/or spaceflight, and while some virulence potentials of several pathogens may be enhanced, other bacterial pathogens could experience a hypovirulence phenotype following exposure to the same conditions. Further, the precise mechanism by which low-shear forces influence bacterial virulence remains largely unknown. Taken together, additional studies aimed at uncovering the unknown mechanisms governing bacterial response’s to physical stress (e.g., low-shear forces) are warranted. Finally, the research community should be mindful of the great disparity observed in various bacterial responses to LSMMG and/or spaceflight and must endeavor to further characterize additional (as-of-yet untested) pathogens’ virulence potentials following exposure to the aforementioned environments. In doing so, a more exhaustive reference resource can be created for astronauts and microbiologists alike.
References
Allen CA, Niesel DW, Torres AG (2008) The effects of low-shear stress on adherent-invasive Escherichia coli. Environ Microbiol 10:1512–1525
Altenburg SD, Nielsen-Preiss SM, Hyman LE (2008) Increased filamentous growth of Candida albicans in simulated microgravity. Genomics Proteomics Bioinforma 6(1):42–50
Balla KM, Troemel ER (2013) Caenorhabditis elegans as a model for intracellular pathogen infection. Cell Microbiol 15(8):1313–1322
Castro VA, Thrasher AN, Healy M, Ott CM, Pierson DL (2004) Microbial characterization during the early habitation of the International Space Station. Microb Ecol 47(2):119–126
Castro SL, Nelman-Gonzalez M, Nickerson CA, Ott CM (2011) Induction of attachment-independent biofilm formation and repression of Hfq expression by low-fluid-shear culture of Staphylococcus aureus. Appl Environ Microbiol 77(18):6368–6378
Chao Y, Vogel J (2010) The role of Hfq in bacterial pathogens. Curr Opin Microbiol 13(1):24–33
Chopra V, Fadl AA, Sha J, Chopra S, Galindo CL, Chopra AK (2006) Alterations in the virulence potential of enteric pathogens and bacterial-host cell interactions under simulated microgravity conditions. J Toxicol Environ Health A 69:1345–1370
Crabbé A, De Boever P, Van Houdt R, Moors H, Mergeay M, Cornelis P (2008) Use of the rotating wall vessel technology to study the effect of shear stress on growth behavior of Pseudomonas aeruginosa PA01. Environ Microbiol 10(8):2098–2110
Crabbé A, Pycke B, Van Houdt R, Monsieurs P, Nickerson C, Leys N, Cornelis P (2010) Response of Pseudomonas aeruginosa PAO1 to low shear modelled microgravity involves AlgU regulation. Environ Microbiol 12(6):1545–1564
Crabbé A, Schurr MJ, Monsieurs P, Morici L, Schurr J, Wilson JW, Ott CM, Tsaprailis G, Pierson DL, Stefanyshyn-Piper H, Nickerson CA (2011) Transcriptional and proteomic responses of Pseudomonas aeruginosa PAO1 to spaceflight conditions involve Hfq regulation and reveal a role for oxygen. Appl Environ Microbiol 77(4):1221–1230
Crabbé A, Nielsen-Preiss SM, Woolley CM, Barrila J, Buchanan K, McCracken J, Inglis DO, Searles SC, Nelman-Gonzalez MA, Ott CM, Wilson JW, Pierson DL, Stefanyshyn-Piper HM, Hyman LE, Nickerson CA (2013) Spaceflight enhances cell aggregation and random budding in Candida albicans. PLoS One 8(12):e80677
Crucian BE, Stowe RP, Pierson DL, Sams CF (2008) Immune system dysregulation following short- vs. long-duration spaceflight. Aviat Space Environ Med 79(9):835–843
Demain AL, Fang A (2001) Secondary metabolism in simulated microgravity. Chem Rec 1(4):333–346
Dornmayr-Pfaffenhuemer M, Legat A, Schwimbersky K, Fendrihan S, Stan-Lotter H (2011) Responses of Haloarchaea to simulated microgravity. Astrobiology 11(3):199–205
Fang A, Pierson DL, Koenig DW, Mishra SK, Demain AL (1997a) Effects of simulated microgravity and shear stress on microcin B17 production by Escherichia coli on its excretion in to the medium. Appl Environ Microbiol 63(10):4090–4092
Fang A, Pierson DL, Mishra SK, Koenig DW, Demain AL (1997b) Secondary metabolism in simulated microgravity: beta lactam production by Streptomyces clavuligerus. J Ind Microbiol Biotechnol 18(1):22–25
Fang A, Pierson DL, Mishra SK, Koenig DW, Demain AL (1997c) Gramicidin S production by Bacillus brevis in simulated microgravity. Curr Microbiol 34(4):199–204
Fang A, Pierson DL, Mishra SK, Demain AL (2000) Growth of Streptomyces hygroscopicus in rotating-wall bioreactor under simulated microgravity inhibits rapamycin production. Appl Microbiol Biotechnol 54(1):33–36
Hamill OP, Martinac B (2001) Molecular basis of mechanotransduction in living cells. Physiol Rev 81(2):685–740
Hammond TG, Stodieck L, Birdsall HH, Becker JL, Koenig P, Hammond JS, Gunter MA, Allen PL (2013) Effects of microgravity on the virulence of Listeria monocytogenes, Enterococcus faecalis, Candida albicans, and Methicillin-Resistant Staphylococcus aureus. Astrobiology 13(11):1081–1090
Ingber D (1999) How cells (might) sense microgravity. FASEB J 13(Suppl:S3):15
Jorgensen JH, Skweres JA, Mishra SK, McElmeel ML, Maher LA, Mulder R, Lancaster MV, Pierson DL (1997) Development of an antimicrobial susceptibility testing method suitable for performance during space flight. J Clin Microbiol 35(8):2093–2097
Juergensmeyer MA, Juergensmeyer EA, Guikema JA (1999) Long-term exposure to spaceflight conditions affect bacterial responses to antibiotics. Microgravity Sci Technol 12(1):41–47
Kacena MA, Todd P (1999) Gentamicin: effect on E. coli in space. Microgravity Sci Technol 12(3–4):135–137
Kaur I, Simons ER, Castro VA, Mark Ott C, Pierson DL (2004) Changes in neutrophil functions in astronauts. Brain Behav Immun 18(5):443–450
Kaur I, Simons ER, Kapadia AS, Ott CM, Pierson DL (2008) Effect of spaceflight on ability of monocytes to respond to endotoxins of Gram-negative bacteria. Clin Vaccine Immunol 15(10):1523–1528
Kim W, Tengra FK, Shong J, Marchand N, Chan HK, Young Z, Pangule RC, Parra M, Dordick JS, Plawsky JL, Collins CH (2013a) Effect of spaceflight on Pseudomonas aeruginosa final cell density is modulated by nutrient and oxygen availability. BMC Microbiol 13:241
Kim W, Tengra FK, Young Z, Shong J, Marchand N, Chan HK, Pangule RC, Parra M, Dordick JS, Plawsky JL, Collins CH (2013b) Spaceflight promotes biofilm formation by Pseudomonas aeruginosa. PLoS One 8(4):e62437
Kim HW, Matin A, Rhee MS (2014) Microgravity alters the physiological characteristics of Escherichia coli O157:H7 ATCC 35150, ATCC 43889, and ATCC 43895 under different nutrient conditions. Appl Environ Microbiol 80(7):2270–2278
Klaus DM, Howard HN (2006) Antibiotic efficacy and microbial virulence during space flight. Trends Biotechnol 24(3):131–136
La Duc MT, Sumner R, Pierson D, Venkat P, Venkateswaran K (2004) Evidence of pathogenic microbes in the International Space Station drinking water: reason for concern? Habitation (Elmsford) 10(1):39–48
Lawal A, Jejelowo OA, Rosenzweig JA (2010) The effects of low-shear mechanical stress on Yersinia pestis virulence. Astrobiology 10(9):881–888
Lawal A, Kirtley ML, van Lier CJ, Erova TE, Kozlova EV, Sha J, Chopra AK, Rosenzweig JA (2013) The effects of modeled microgravity on growth kinetics, antibiotic susceptibility, cold growth, and the virulence potential of a Yersinia pestis ymoA-deficient mutant and its isogenic parental strain. Astrobiology 13(9):821–832
Lynch SV, Brodie EL, Matin A (2004) Role and regulation of σs in general resistance conferred by low shear simulated microgravity in Escherichia coli. J Bacteriol 186(24):8207–8212
Lynch SV, Mukundakrishnan K, Benoit MR, Ayyaswamy PS, Matin A (2006) Escherichia coli biofilms formed under low shear modeled microgravity in a ground-based system. Appl Environ Microbiol 72:7701–7710
Maule J, Wainwright N, Steele A, Monaco L, Morris H, Gunter D, Damon M, Wells M (2009) Rapid culture-independent microbial analysis aboard the International Space Station (ISS). Astrobiology 9(8):759–775
McLean RJ, Cassanto JM, Barnes MB, Koo JH (2001) Bacterial biofilm formation under microgravity conditions. FEMS Microbiol Lett 195(2):115–119
Nickerson CA, Ott CM, Mister SJ, Morrow BJ, Burns-Kelicher L, Pierson DL (2000) Microgravity as a novel environmental signal affecting Salmonella enterica typhimurium virulence. Infect Immun 68:3147–3152
Nickerson CA, Ott CM, Wilson JW, Ramamurthy R, LeBlanc CL, zu Honer BK, Hammond T, Pierson DL (2003) Low-shear modeled microgravity: a global environmental regulatory signal affecting bacterial gene expression, physiology, and pathogenesis. J Microbiol Methods 54:1–11
Nickerson CA, Ott CM, Wilson JW, Ramamurthy R, Pierson DL (2004) Microbial responses to microgravity and other low-shear environments. Microbiol Mol Biol Rev 68(2):345–361
Norman MU, Moriarty TJ, Dresser AR, Millen B, Kubes P, Chaconas G (2008) Molecular mechanisms involved in vascular interactions of the Lyme disease pathogen in a living host. PLoS Pathog 4(10):e1000169
Pacello F, Rotilio G, Battistoni A (2012) Low shear modelled microgravity enhances Salmonella enterica resistance to hydrogen peroxide through a mechanism involving KatG and KatN. Open Microbiol J 6:53–64
Purevdorj-Gage B, Sheehan KB, Hyman LE (2006) Effects of low-shear modeled microgravity on cell function, gene expression, and phenotype in Saccharomyces cerevisiae. Appl Environ Microbiol 72:4569–4575
Rosenzweig JA, Abogunde O, Thomas K, Lawal A, Nguyen YU, Sodipe A, Jejelowo O (2010) Spaceflight and modeled microgravity effects on microbial growth and virulence. Appl Microbiol Biotechnol 85(4):885–891
Searless SC, Woolley CM, Petersen RA, Hyman LE, Nielsen-Preiss SM (2011) Modeled microgravity increases filamentation, biofilm formation, phenotypic switching, and antimicrobial resistance in Candida albicans. Astrobiology 11(8):825–836
Sheehan KB, McInnerney K, Purevdorj-Gage B, Altenburg SD, Hyman LE (2007) Yeast genomic expression patterns in response to low-shear modeled microgravity. BMC Genomics 8:3
Soni A, O’Sullivan L, Quick LN, Ott CM, Nickerson CA, Wilson JW (2014) Conservation of the Low-shear modeled microgravity response in Enterobacteriaceae and analysis of the trp genes in this response. Open Microbiol J 8:51–58
Spencer H, Karavolos MH, Bulmer DM, Aldridge P, Chhabra SR, Winzer K, Williams P, Khan CM (2010) Genome-wide transposon mutagenesis identifies a role for host neuroendocrine stress hormones in regulating the expression of virulence genes in Salmonella. J Bacteriol 192(3):714–724
Stowe RP, Kozlova EV, Sams CF, Pierson DL, Walling DM (2011) Latent and lytic Epstein-Barr virus gene expression in the peripheral blood of astronauts. J Med Virol 83(6):1071–1077. doi:10.1002/jmv.22079
Thomas WE, Trintchina E, Forero M, Vogel V, Sokurenko EV (2002) Bacterial adhesion to target cells enhanced by shear force. Cell 109(7):913–923
Tixador R, Richoilley G, Gasset G, Templier J, Bes JC, Moatti N, Lapchine L (1985) Study of minimal inhibitory concentration of antibiotics on bacteria cultivated in vitro in space (Cytos 2 experiment). Aviat Space Environ Med 56(8):748–751
Tucker DL, Ott CM, Huff S, Fofanov Y, Pierson DL, Wilson RC, Fox GE (2007) Characterization of E. coli MG1655 grown in a low-shear modeled microgravity environment. BMC Microbiol 7:15
Vesper SJ, Wong W, Kuo CM, Pierson DL (2008) Mold species in dust from the International Space Station identified and quantified by mold-specific quantitative PCR. Res Microbiol 159(6):432–435
Wilson JW, Ramamurthy R, Porwollik S, McClelland M, Hammond T, Allen P, Ott CM, Pierson DL, Nickerson CA (2002a) Microarray analysis identifies Salmonella genes belonging to the low-shear modeled microgravity regulon. Proc Natl Acad Sci U S A 99:13807–13812
Wilson J, Ott CM, Ramamurthy R, Porwollik S, McClelland M, Pierson DL, Nickerson CA (2002b) Low shear modeled microgravity alters the Salmonella enterica serovar typhimurium stress response in an RpoS-independent manner. Appl Environ Microbiol 68:5408–5416
Wilson JW, Ott CM, zu Höner BK, Ramamurthy R, Quick L, Porwollik S, Cheng P, McClelland M, Tsaprailis G, Radabaugh T, Hunt A, Fernandez D, Richter E, Shah M, Kilcoyne M, Joshi L, Nelman-Gonzalez M, Hing S, Parra M, Dumars P, Norwood K, Bober R, Devich J, Ruggles A, Goulart C, Rupert M, Stodieck L, Stafford P, Catella L, Schurr MJ, Buchanan K, Morici L, McCracken J, Allen P, Baker-Coleman C, Hammond T, Vogel J, Nelson R, Pierson DL, Stefanyshyn-Piper HM, Nickerson CA (2007) Space flight alters bacterial gene expression and virulence and reveals a role for global regulator Hfq. Proc Natl Acad Sci 104:16299–16304
Wilson JW, Ott CM, Quick L, Davis R, zu Höner BK, Crabbé A, Richter E, Sarker S, Barrila J, Porwollik S, Cheng P, McClelland M, Tsaprailis G, Radabaugh T, Hunt A, Shah M, Nelman-Gonzalez M, Hing S, Parra M, Dumars P, Norwood K, Bober R, Devich J, Ruggles A, CdeBaca A, Narayan S, Benjamin J, Goulart C, Rupert M, Catella L, Schurr MJ, Buchanan K, Morici L, McCracken J, Porter MD, Pierson DL, Smith SM, Mergeay M, Leys N, Stefanyshyn-Piper HM, Gorie D, Nickerson CA (2008) Media ion composition controls regulatory and virulence response of Salmonella in spaceflight. PLoS One 3:e3923
Acknowledgments
We would like to greatly acknowledge the lively discussions and insights provided by Shishir Shishodia, George Fox, Hector Miranda, Ayodotun Sodipe, Duane L. Pierson, and C. Mark Ott. Work on this manuscript was supported by National Aeronautics and Space Administration (NASA) cooperative agreement NNX08B4A47A (JAR), and NIH/NIAID AI064389 and N01 AI30065 grants awarded to AKC.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rosenzweig, J.A., Ahmed, S., Eunson, J. et al. Low-shear force associated with modeled microgravity and spaceflight does not similarly impact the virulence of notable bacterial pathogens. Appl Microbiol Biotechnol 98, 8797–8807 (2014). https://doi.org/10.1007/s00253-014-6025-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-6025-8