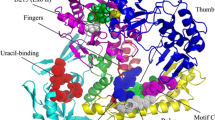
Abstract
During the genomics era, the use of thermostable DNA polymerases increased greatly. Many were identified and described—mainly of the genera Thermus, Thermococcus and Pyrococcus. Each polymerase has different features, resulting from origin and genetic modification. However, the rational choice of the adequate polymerase depends on the application itself. This review gives an overview of the most commonly used DNA polymerases used for PCR application: KOD, Pab (Isis™), Pfu, Pst (Deep Vent™), Pwo, Taq, Tbr, Tca, Tfi, Tfl, Tfu, Tgo, Tli (Vent™), Tma (UITma™), Tne, Tth and others.
Similar content being viewed by others
References
Al-Soud WA, Rådström P (1998) Capacity of nine thermostable DNA polymerases to mediate DNA amplification in the presence of PCR-inhibiting samples. Appl Environ Microbiol 64:3748–3753
Al-Soud WA, Rådström P (2000) Effects of amplification facilitators on diagnostic PCR in the presence of blood, feces, and meat. J Clin Microbiol 38:4463–4470
Al-Soud WA, Rådström P (2001) Purification and characterization of PCR-inhibitory components in blood cells. J Clin Microbiol 39:485–493
André P, Kim A, Khrapko K, Thilly WG (1997) Fidelity and mutational spectrum of Pfu DNA polymerase on a human mitochondrial DNA sequence. Genome Res 7:843–852
Angers M, Cloutier JF, Castonguay A, Drouin R (2001) Optimal conditions to use Pfu exo(−) DNA polymerase for highly efficient ligation-mediated polymerase chain reaction protocols. Nucleic Acids Res 29:E83
Arezi B, Xing W, Sorge JA, Hogrefe HH (2003) Amplification efficiency of thermostable DNA polymerases. Anal Biochem 321:226–235
Atomi H, Fukui T, Kanai T, Morikawa M, Imanaka T (2004) Description of Thermococcuskodacaraensis sp. nov., a well studied hyperthermophilic archaeon previously reported as Pyrococcus sp. KOD1. Archaea 1:263–267
Bae H, Kim KP, Lee JI, Song JG, Kil EJ, Kim JS, Kwon ST (2009) Characterization of DNA polymerase from the hyperthermophilic archaeon Thermococcus marinus and its application to PCR. Extremophiles 13:657–667
Barnes WM (1994) PCR amplification of up to 35-kb DNA with high fidelity and high yield from lambda bacteriophage templates. Proc Natl Acad Sci 91:2216–2220
Birch DE, Laird WJ, Zoccoli A (1998) Nucleic acid amplification using a reversibly inactivated thermostable enzyme. Patent appl no US 5773258
Böhlke K, Pisani FM, Vorgias CE, Frey B, Sobek H, Rossi M, Antranikian G (2000) PCR performance of the B-type DNA polymerase from the thermophilic euryarchaeon Thermococcus aggregans improved by mutations in the Y-GG/A motif. Nucleic Acids Res 28:3910–3917
Bonch-Osmolovskaya E, Svetlichny V, Ankenbauer W, Schmitz-Agheguian G, Angerer B, Ebenbichler C, Laue F (1996) Thermostable nucleic acid polymerase from Thermococcus gorgonarius. Patent appl no EP 0834570 A1
Braithwaite DK, Ito J (1993) Compilation, alignment, and phylogenetic relationships of DNA polymerases. Nucleic Acids Res 21:787–802
Callen W, Mathur EJ (1999) Isolation and identification of polymerases. Patent US 5948666A
Cambon-Bonavita MA, Schmitt P, Zieger M, Flaman JM, Lesongeur F, Raguénès G, Bindel D, Frisch N, Lakkis Z, Dupret D, Barbier G, Quérellou J (2000) Cloning, expression, and characterization of DNA polymerase I from the hyperthermophilic archaea Thermococcus fumicolans. Extremophiles 4:215–225
Cann IK, Ishino S, Nomura N, Sako Y, Ishino Y (1999) Two family B DNA polymerases from Aeropyrum pernix, an aerobic hyperthermophilic crenarchaeote. J Bacteriol 181:5984–5992
Carballeira N, Nazabal M, Brito J, Garcia O (1990) Purification of a thermostable DNA polymerase from Thermus thermophilus HB8, useful in the polymerase chain reaction. Biotechniques 9:276–281
Cariello NF, Swenberg JA, Skopek TR (1991) Fidelity of Thermococcus litoralis DNA polymerase (Vent) in PCR determined by denaturing gradient gel electrophoresis. Nucleic Acids Res 19:4193–4198
Chang M, Lee HJ (2005) Gradient polymerase chain reaction performance using regular thermal cycle machine. Anal Biochem 340:174–177
Chaput JC, Szostak JW (2003) TNA synthesis by DNA polymerases. J Am Chem Soc 125:9274–9275
Chatterjee DK, Potomac N (1999) Cloned DNA polymerases from Thermotoga maritima and mutants thereof. Patent appl no US 5948614
Chatterjee DK, Potomac N, Hughes J (2002) Cloned DNA polymerases from Thermotoga neopolitana. Patent appl no US 6444424B1
Cheng S, Fockler C, Barnes WM, Higuchi R (1994) Effective amplification of long targets from cloned inserts and human genomic DNA. Proc Natl Acad Sci 91:5695–5699
Chester N, Marshak DR (1993) Dimethyl sulfoxide-mediated primer Tm reduction: a method for analyzing the role of renaturation temperature in the polymerase chain reaction. Anal Biochem 209:284–290
Chien A, Edgar DB, Trela JM (1976) Deoxyribonucleic acid polymerase from the extreme thermophile Thermus aquaticus. J Bact 127:1550–1557
Cho SS, Kim KP, Lee KK, Youn MH, Kwon ST (2012) Characterization and PCR application of a new high-fidelity DNA polymerase from Thermococcus waiotapuensis. Enzyme Microb Technol 51:334–341
Choi JJ, Jung SE, Kim HK, Kwon ST (1999) Purification and properties of Thermus filiformis DNA polymerase expressed in Escherichia coli. Biotechnol Appl Biochem 30:19–25
Chong SS, Eichler EE, Nelson DL, Hughes MR (1994) Robust amplification and ethidium-visible detection of the fragile X syndrome CGG repeat using Pfu polymerase. Am J Med Genet 51:522–526
Chou Q, Russell M, Birch DE, Raymond J, Bloch W (1992) Prevention of pre-PCR mis-priming and primer dimerization improves low-copy-number amplifications. Nucleic Acids Res 20:1717–1723
Cline J, Braman JC, Hogrefe HH (1996) PCR fidelity of pfu DNA polymerase and other thermostable DNA polymerases. Nucleic Acids Res 24:3546–3551
Dabrowski S, Kiaer Ahring B (2003) Cloning, expression, and purification of the His6-tagged hyper-thermostable dUTPase from Pyrococcus woesei in Escherichia coli: application in PCR. Protein Expr Purif 31:72–78
Dabrowski S, Kur J (1998) Cloning and expression in Escherichia coli of the recombinant his-tagged DNA polymerases from Pyrococcus furiosus and Pyrococcus woesei. Protein Expr Purif 14:131–138
Dang C, Jayasena SD (1996) Oligonucleotide inhibitors of Taq DNA polymerase facilitate detection of low copy number targets by PCR. J Mol Biol 264:268–278
Datukishvili N, Pokholok D, Lottspeich F, Prangishvili D, Rechinsky V (1996) The DNA polymerase-encoding gene from a thermoacidophilic archaeon Sulfolobus acidocaldarius. Gene 177:271–273
de Noronha CM, Mullins JI (1992) Amplimers with 3′-terminal phosphorothioate linkages resist degradation by vent polymerase and reduce Taq polymerase mispriming. PCR Methods Appl 2:131–136
Diaz RS, Sabino EC (1998) Accuracy of replication in the polymerase chain reaction. Comparison between Thermotoga maritima DNA polymerase and Thermus aquaticus DNA polymerase. Braz J Med Biol Res 31:1239–1242
Dietrich J, Schmitt P, Zieger M, Preve B, Rolland JL, Chaabihi H, Gueguen Y (2002) PCR performance of the highly thermostable proof-reading B-type DNA polymerase from Pyrococcus abyssi. FEMS Microbiol Lett 217:89–94
Eckert KA, Kunkel TA (1990) High fidelity DNA synthesis by the Thermus aquaticus DNA polymerase. Nucleic Acids Res 18:3739–3744
Fiala G, Stetter KO (1986) Pyrococcus furiosus sp. nov. represents a novel genus of marine heterotrophic archaebacteria growing optimally at 100 °C. Arch Microbiol 145:56–61
Flaman JM, Frebourg T, Moreau V, Charbonnier F, Martin C, Ishioka C, Friend SH, Iggo R (1994) A rapid PCR fidelity assay. Nucleic Acids Res 22:3259–3260
Gelfand GH, Lawyer FC, Stoffel S (1995) Mutated thermostable nucleic acid polymerase enzyme from Thermotoga maritima. Patent appl no US 5420029
Ghasemi A, Salmanian AH, Sadeghifard N, Salarian AA, Gholi MK (2011) Cloning, expression and purification of Pwo polymerase from Pyrococcus woesei. Iran J Microbiol 3:118–122
Griffiths K, Nayak S, Park K, Mandelman D, Modrell B, Lee J, Ng B, Gibbs MD, Bergquist PL (2007) New high fidelity polymerases from Thermococcus species. Protein Expr Purif 52:19–30
Gueguen Y, Rolland JL, Lecompte O, Azam P, Le Romancer G, Flament D, Raffin JP, Dietrich J (2001) Characterization of two DNA polymerases from the hyperthermophilic euryarchaeon Pyrococcus abyssi. Eur J Biochem 268:5961–5969
Harrell RA 2nd, Hart RP (1994) Rapid preparation of Thermus flavus DNA polymerase. PCR Methods Appl 3:372–375
Henke W, Herdel K, Jung K, Schnorr D, Loening SA (1997) Betaine improves the PCR amplification of GC-rich DNA sequences. Nucleic Acids Res 25:3957–3958
Herrin BR, Groeger AL, Justement LB (2005) The adaptor protein HSH2 attenuates apoptosis in response to ligation of the B cell antigen receptor complex on the B lymphoma cell line, WEHI-231. J Biol Chemm 280:3507–3515
Hogrefe HH, Hansen CJ, Scott BR, Nielson KB (2002) Archaeal dUTPase enhances PCR amplifications with archaeal DNA polymerases by preventing dUTP incorporation. Proc Natl Acad Sci 99:596–601
Holton TA, Graham MW (1991) A simple and efficient method for direct cloning of PCR products using ddT-tailed vectors. Nucleic Acids Res 19:1156
Huang YP, Ito J (1998) The hyperthermophilic bacterium Thermotoga maritima has two different classes of family C DNA polymerases: evolutionary implications. Nucleic Acids Res 26:5300–5309
Huang H, Keohavong P (1996) Fidelity and predominant mutations produced by deep vent wild-type and exonuclease-deficient DNA polymerases during in vitro DNA amplification. DNA Cell Biol 15:589–594
Huber R, Langworthy TA, König H, Thomm M, Woese CR, Sleytr UB, Stetter KO (1986) Thermotoga maritima sp. nov. represents a new genus of unique extremely thermophilic eubacteria growing up to 90 °C. Arch Microbiol 144:324–333
Ikehara Y, Ikehara SK, Paulson JC (2004) Negative regulation of T cell receptor signaling by Siglec-7 (p70/AIRM) and Siglec-9. J Biol Chem 279:43117–43125
Jannasch HW, Wirsen CO, Molyneaux SJ, Langworthy TA (1992) Comparative physiological studies on hyperthermophilic archaea isolated from deep-sea hot vents with emphasis on Pyrococcus strain GB-D. Appl Environ Microbiol 58:3472–3481
Kähler M, Antranikian G (2000) Cloning and characterization of a family B DNA polymerase from the hyperthermophilic crenarchaeon Pyrobaculum islandicum. J Bacteriol 182:655–663
Kaledin AS, Sliusarenko AG, Gorodetskiĭ SI (1980) Isolation and properties of DNA polymerase from extreme thermophylic bacteria Thermus aquaticus YT-1. Biokhimiia 45:644–651, Russian
Kellog DE, Rybalkin I, Chen S, Mukhamedova N, Vlasik T, Siebert PD, Chenchik A (1994) TaqStart Antibody: “hot start” PCR facilitated by a neutralizing monoclonal antibody directed against Taq DNA polymerase. Biotechniques 16:1134–1137
Kennedy EM, Hergott C, Dewhurst S, Kim B (2009) The mechanistic architecture of thermostable Pyrococcus furiosus family B DNA polymerase motif A and its interaction with the dNTP substrate. Biochemistry 48:11161–11168
Keohavong P, Thilly WG (1989) Fidelity of DNA polymerases in DNA amplification. Proc Natl Acad Sci 86:9253–9257
Kermekchiev MB, Tzekov A, Barnes WM (2003) Cold-sensitive mutants of Taq DNA polymerase provide a hot start for PCR. Nucl Acids Res 31:6139–6147
Kim S, Labbe RG, Ryu S (2000) Inhibitory effects of collagen on the PCR for detection of Clostridium perfringens. Appl Environ Microbiol 66:1213–1215
Kim TS, Maeda A, Maeda T, Heinlein C, Kedishvili N, Palczewski K, Nelson PS (2005) Delayed dark adaptation in 11-cis-retinol dehydrogenase-deficient mice: a role of RDH11 in visual processes in vivo. J Biol Chem 280:8694–8704
Kim YJ, Lee HS, Bae SS, Jeon JH, Lim JK, Cho Y, Nam KH, Kang SG, Kim SJ, Kwon ST, Lee JH (2007) Cloning, purification, and characterization of a new DNA polymerase from a hyperthermophilic archaeon, Thermococcus sp. NA1. J Microbiol Biotechnol 17:1090–1097
Kim KP, Bae H, Kim IH, Kwon ST (2011) Cloning, expression, and PCR application of DNA polymerase from the hyperthermophilic archaeon, Thermococcus celer. Biotechnol Lett 33:339–346
Kong H, Kucera RB, Jack WE (1993) Characterization of a DNA polymerase from the hyperthermophile archaea Thermococcus litoralis. Vent DNA polymerase, steady state kinetics, thermal stability, processivity, strand displacement, and exonuclease activities. J Biol Chem 268:1965–1975
Lawyer FC, Stoffel S, Saiki RK, Chang SY, Landre PA, Abramson RD, Gelfand DH (1993) High-level expression, purification, and enzymatic characterization of full-length Thermus aquaticus DNA polymerase and a truncated form deficient in 5′ to 3′ exonuclease activity. PCR Methods Appl 2:275–287
Lee JI, Kim YJ, Bae H, Cho SS, Lee J, Kwon S (2010) Biochemical properties and PCR performance of a family B DNA polymerase from hyperthermophilic Euryarchaeon Thermococcus peptonophilus. Appl Biochem Biotech 160:1585–1599
Ling LL, Keohavong P, Dias C, Thilly WG (1991) Optimization of the polymerase chain reaction with regard to fidelity: modified T7, Taq, and Vent DNA polymerases. PCR Methods Appl 1:63–69
Lu C, Erickson HP (1997) Expression in Escherichia coli of the thermostable DNA polymerase from Pyrococcus furiosus. Protein Expr Purif 11:179–184
Lundberg KS, Shoemaker DD, Adams MW, Short JM, Sorge JA, Mathur EJ (1991) High-fidelity amplification using a thermostable DNA polymerase isolated from Pyrococcus furiosus. Gene 108:1–6
Marsic D, Flaman JM, Ng JD (2008) New DNA polymerase from the hyperthermophilic marine archaeon Thermococcus thioreducens. Extremophiles 12:775–788
Masud MM, Ozaki-Nakamura A, Satou F, Ohbayashi T, Ozaki H, Sawai H (2001) Enzymatic synthesis of modified DNA by PCR. Nucleic Acids Res Suppl 1:21–22
Mattila P, Korpela J, Tenkanen T, Pitkänen K (1991) Fidelity of DNA synthesis by the Thermococcus litoralis DNA polymerase—an extremely heat stable enzyme with proofreading activity. Nucleic Acids Res 19:4967–4973
McDonald JP, Hall A, Gasparutto D, Cadet J, Ballantyne J, Woodgate R (2006) Novel thermostable Y-family polymerases: applications for the PCR amplification of damaged or ancient DNAs. Nucleic Acids Res 34:1102–1111
Miroshnichenko ML, Gongadze GM, Rainey FA, Kostyukova AS, Lysenko AM, Chernyh NA, Bonch-Osmolovskaya EA (1998) Thermococcus gorgonarius sp. nov. and Thermococcus pacificus sp. nov.: heterotrophic extremely thermophilic archaea from New Zealand submarine hot vents. Int J Syst Bacteriol 48:23–29
Mizuguchi H, Nakatsuji M, Fujiwara S, Takagi M, Imanaka T (1999) Characterization and application to hot start PCR of neutralizing monoclonal antibodies against KOD DNA polymerase. Biochem 126:762–768
Moretti T, Koons B, Budowle B (1998) Enhancement of PCR amplification yield and specificity using AmpliTaq Gold DNA polymerase. Biotechniques 25:716–722
Neuner A, Jannasch HW, Belkin S, Stetter KO (1990) Thermococcus litoralis sp. nov.: a new species of extremely thermophilic marine archaebacteria. Arch Microbiol 153:205–207
Nishioka M, Mizuguchi H, Fujiwara S, Komatsubara S, Kitabayashi M, Uemura H, Takagi M, Imanaka T (2001) Long and accurate PCR with a mixture of KOD DNA polymerase and its exonuclease deficient mutant enzyme. J Biotechnol 88:141–149
Nisole S, Lynch C, Stoye JP, Yap MW (2004) A Trim5-cyclophilin A fusion protein found in owl monkey kidney cells can restrict HIV-1. Proc Natl Acad Sci 101:13324–13328
Owczarzy R, Moreira BG, You Y, Behlke MA, Walder JA (2008) Predicting stability of DNA duplexes in solutions containing magnesium and monovalent cations. Biochemistry 47:5336–5353
Park JH, Kim JS, Kwon ST, Lee DS (1993) Purification and characterization of Thermus caldophilus GK24 DNA polymerase. Eur J Biochem 214:135–140
Paul N, Shum J, Le T (2010) Hot start PCR. Methods Mol Biol 630:301–318
Ppyun H, Kim I, Cho SS, Seo KJ, Yoon K, Kwon ST (2012) Improved PCR performance using mutant Tpa-S DNA polymerases from the hyperthermophilic archaeon Thermococcus pacificus. J Biotechnol 164:363–370
Priyakumar UD, Ramakrishna S, Nagarjuna KR, Reddy SK (2010) Structural and energetic determinants of thermal stability and hierarchical unfolding pathways of hyperthermophilic proteins, Sac7d and Sso7d. J Phys Chem B 114:1707–1718
Rees WA, Yager TD, Korte J, von Hippel PH (1993) Betaine can eliminate the base pair composition dependence of DNA melting. Biochemistry 32:137–144
Rual JF, Hirozane-Kishikawa T, Hao T, Bertin N, Li S, Dricot A, Li N, Rosenberg J, Lamesch P, Vidalain PO, Clingingsmith TR, Hartley JL, Esposito D, Cheo D, Moore T, Simmons B, Sequerra R, Bosak S, Doucette-Stamm L, Le Peuch C, Vandenhaute J, Cusick ME, Albala JS, Hill DE, Vidal M (2004) Human ORFeome version 1.1: a platform for reverse proteomics. Genome Res 14:2128–2135
Rüttimann C, Cotorás M, Zaldívar J, Vicuña R (1985) DNA polymerases from the extremely thermophilic bacterium Thermus thermophilus HB-8. Eur J Biochem 149:41–46
Saiki RK, Gelfand DH, Stoffel S, Scharf SJ, Higuchi R, Horn GT, Mullis KB, Erlich HA (1988) Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science 239:487–491
Sarkar G, Kapelner S, Sommer SS (1990) Formamide can dramatically improve the specificity of PCR. Nucleic Acids Res 18:7465
Sasaki Y, Casola S, Kutok JL, Rajewsky K, Schmidt-Supprian M (2004) TNF family member B cell-activating factor (BAFF) receptor-dependent and -independent roles for BAFF in B cell physiology. J Immunol 173:2245–2252
Sawai H, Ozaki-Nakamura A, Mine M, Ozaki H (2002) Synthesis of new modified DNAs by hyperthermophilic DNA polymerase: substrate and template specificity of functionalized thymidine analogues bearing an sp3-hybridized carbon at the C5 alpha-position for several DNA polymerases. Bioconjug Chem 13:309–316
Schoder D, Schmalwieser A, Schauberger G, Kuhn M, Hoorfar J, Wagner M (2003) Physical characteristics of six new thermocyclers. Clin Chem 49:960–963
Schoder D, Schmalwieser A, Schauberger G, Hoorfar J, Kuhn M, Wagner M (2005) Novel approach for assessing performance of PCR cyclers used for diagnostic testing. J Clin Microbiol 43:2724–2728
Sharkey DJ, Scalice ER, Christy KG Jr, Atwood SM, Daiss JL (1994) Antibodies as thermolabile switches: high temperature triggering for the polymerase chain reaction. Biotechnology 12:506–509
Skerra A (1992) Phosphorothioate primers improve the amplification of DNA sequences by DNA polymerases with proofreading activity. Nucleic Acids Res 20:3551–3554
Slupphaug G, Alseth I, Eftedal I, Volden G, Krokan HE (1993) Low incorporation of dUMP by some thermostable DNA polymerases may limit their use in PCR amplifications. Anal Biochem 211:164–169
Takagi M, Nishioka M, Kakihara H, Kitabayashi M, Inoue H, Kawakami B, Oka M, Imanaka T (1997) Characterization of DNA polymerase from Pyrococcus sp. Strain KOD1 and its application to PCR. Appl Environ Microbiol 63:4504–4510
Tindall KR, Kunkel TA (1988) Fidelity of DNA synthesis by the Thermus aquaticus DNA polymerase. Biochemistry 27:6008–6013
Trower MK, Elgar GS (1994) PCR cloning using T-vectors. Methods Mol Biol 31:19–33
Uemori T, Ishino Y, Doi H, Kato I (1995) The hyperthermophilic archaeon Pyrodictium occultum has two alpha-like DNA polymerases. J Bacteriol 177:2164–2177
Varadaraj K, Skinner DM (1994) Denaturants or cosolvents improve the specificity of PCR amplification of a G + C-rich DNA using genetically engineered DNA polymerases. Gene 140:1–5
Vigneault F, Drouin R (2005) Optimal conditions and specific characteristics of Vent exo − DNA polymerase in ligation-mediated polymerase chain reaction protocols. Biochem Cell Biol 83:147–165
von Ahsen N, Wittwer CT, Schütz E (2001) Oligonucleotide melting temperatures under PCR conditions: nearest-neighbor corrections for Mg(2+), deoxynucleotide triphosphate, and dimethyl sulfoxide concentrations with comparison to alternative empirical formulas. Clin Chem 47:1956–1961
Wang Y, Prosen DE, Mei L, Sullivan JC, Finney M, Vander Horn PB (2004) A novel strategy to engineer DNA polymerases for enhanced processivity and improved performance in vitro. Nucleic Acids Res 32:1197–1207
Wang Y, Horn PV, Xi L (2012) Methods of using improved polymerases. US patent 20120295270
Wiedbrauk DL, Werner JC, Drevon AM (1995) Inhibition of PCR by aqueous and vitreous fluids. J Clin Microbiol 33:2643–2646
Windberger E, Huber R, Trincone A, Fricke R, Stetter K (1989) Thermotoga thermarum sp. nov. and Thermotoga neapolitana occurring in African continental solfataric springs. Arch Microbiol 151:506–512
Wu DY, Ugozzoli L, Pal BK, Qian J, Wallace RB (1991) The effect of temperature and oligonucleotide primer length on the specificity and efficiency of amplification by the polymerase chain reaction. DNA Cell Biol 10:233–238
Wu G, Wolf JB, Ibrahim AF, Vadasz S, Gunasinghe M, Freeland SJ (2006) Simplified gene synthesis: a one-step approach to PCR-based gene construction. J Biotechnol 124:496–503
Yang SW, Astatke M, Potter J, Chatterjee DK (2002) Mutant Thermotoga neapolitana DNA polymerase I: altered catalytic properties for non-templated nucleotide addition and incorporation of correct nucleotides. Nucleic Acids Res 30:4314–4320
Yu K, Pauls KP (1992) Optimization of the PCR program for RAPD analysis. Nucleic Acids Res 20:2606
Zhang Z, Yang X, Meng L, Liu F, Shen C, Yang W (2009) Enhanced amplification of GC-rich DNA with two organic reagents. Biotechniques 47:775–779
Zheng W, Lee JE, Potter RJ, Mandelman D (2008) DNA polymerase blends and mutant DNA polymerases. Patent appl no 20080254525
Zhou MY, Gomez-Sanchez CE (2000) Universal TA cloning. Curr Issues Mol Biol 2:1–7
Zillig W, Holz I, Klenk HP, Trent J, Wunderl S, Janekovic D, Imsel E, Haas B (1987) Pyrococcus woesei, sp. nov., an ultra-thermophilic marine archaebacterium, representing a novel order, Thermococcales. System App Microbiol 9:62–70
Acknowledgments
The author thanks Prof. A. Steinbüchel for his support this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Terpe, K. Overview of thermostable DNA polymerases for classical PCR applications: from molecular and biochemical fundamentals to commercial systems. Appl Microbiol Biotechnol 97, 10243–10254 (2013). https://doi.org/10.1007/s00253-013-5290-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-013-5290-2