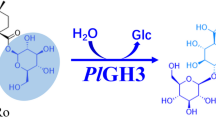
Abstract
The microbial transformation of a series of tetrahydroprotoberberines (THPBs, 1–5) by Gliocladium deliquescens NRRL1086 was investigated. In this research, the novel glycosylation of tetrahydroberberrubine (1) was observed with fast rate and high regio- and enantio-selectivity. One pair of unique enantiomorphic alkaloidal glycosides T-1 and T-2 was isolated and their structures were elucidated unambiguously by HR-MS, CD, 1D and 2D NMR spectrum. It is interesting that different amounts of glucose in the potato broth medium could influence the ratio of T-1 and T-2; in the 1.5% glucose medium, the ratio was about 15:1 and the yield of the S-form product T-1 may reach the theoretical maximum yield of about 50% which could provide one practical method to prepare the enantiomerically pure product and one alternative resolution method of tetrahydroberberrubine. The preliminary enzymatic research by using sodium dodecyl sulfate (SDS) and imidazole as glycosyltransferase and glycosidase inhibitors revealed that glycosyltransferase may contribute to glycosylation process. This is the first successful approach to glycosylation of tetrahydroprotoberberines.






Similar content being viewed by others
References
Betts RE, Walters DE, Rosazza JPN (1974) Microbial transformations of antitumor compounds. 1. Conversion of acronycine to 9-hydroxyacronycine by Cunninghamella echinulata. J Med Chem 17(6):599–602
Chen ND, Zhang J, Liu JH, Yu BY (2010) Microbial conversion of ruscogenin by Gliocladium deliquescens NRRL1086: glycosylation at C-1. Appl Microbiol Biotechnol 86(2):491–497
Chu HY, Jin GZ, Eitan F, Zhen XC (2008) Recent development in studies of tetrahydroprotoberberines: mechanism in antinociception and drug addiction. Cell Mol Neurobiol 28(4):491–499
Clardy J, Walsh C (2004) Lessons from natural molecules. Nature 432(7019):829–837
de Carvalho CCCR (2011) Enzymatic and whole cell catalysis: Finding new strategies for old processes. Biotechnol Adv 29(1):75–83
Dobson CM (2004) Chemical space and biology. Nature 432(7019):824–828
Emidio VLD, Ivana MMF, Diego NG (2005) The alkaloids: chemistry and biology. Academic: New York 62:1–60
Giri A, Dhingra V, Giri CC, Singh A, Ward OP, Narasu ML (2001) Biotransformations using plant cells, organ cultures and enzyme systems: current trends and future prospects. Biotechnol Adv 19(3):175–199
Hays WS, Vanderjagt DJ, Bose B, Serianni AS, Glew RH (1998) Catalytic mechanism and specificity for hydrolysis and transglycosylation reactions of cytosolic β-glucosidase from guinea pig liver. J Biol Chem 273(52):34941–34948
Hu SM, Xu SX, Yao XS, Cui CB, Yasuhiro T, Tohru K (1993) Dauricoside, a new glycosidal alkaloid having an inhibitory activity against blood-platelet aggregation. Chem Pharm Bull 41(10):1866–1868
Iwasa K, Kamigauchi M, Ueki M, Taniguchi M (1996) Antibacterial activity and structure–activity relationships of berberine analogs. Eur J Med Chem 31(6):469–478
Iwasa K, Cui W, Takahashi T, Nishiyama Y, Kamigauchi M, Koyama J, Takeuchi A, Moriyasu M, Takeda K (2010) Biotransformation of phenolic tetrahydroprotoberberines in plant cell cultures followed by LC-NMR, LC-MS, and LC-CD. J Nat Prod 73(2):115–122
Koeller KM, Wong CH (2000) Synthesis of complex carbohydrates and glycoconjugates: enzyme-based and programmable one-pot strategies. Chem Rev 100(12):4465–4493
Langenhan JM, Thorson JS (2005) Recent carbohydrate-based bhemoselective ligation applications. Curr Org Synth 2(1):59–81
Li W, Koike K, Asada Y, Yoshikawa T, Nikaido T (2002) Biotransformation of umbelliferone by Panax ginseng root cultures. Tetrahedron Lett 43(32):5633–5635
Li YH, Yang P, Kong WJ, Wang YX, Hu CQ, Zuo ZY, Wang YM, Gao H, Gao LM, Feng YC, Du NN, Liu Y, Song DQ, Jiang JD (2009) Berberine analogues as a novel class of the low-density-lipoprotein receptor up-regulators: synthesis, structure-activity relationships, and cholesterol-lowering efficacy. J Med Chem 52(2):492–501
Mulder GJ (1992) Glucuronidation and its role in regulation of biological activity of drugs. Annu Rev Pharmacol Toxicol 32(1):25–49
Rantwijk F, Oosterom MW, Sheldon RA (1999) Glycosidase catalysed synthesis of alkyl glycosides. J Mol Catal B: Enzym 6(6):511–532
Ritchie GE, Moffatt BE, Sim RB, Morgan BP, Dwek RA, Rudd PM (2002) Glycosylation and the complement system. Chem Rev 102(2):305–319
Thorson JS, Hosted TJ Jr, Jiang J, Biggins JB, Ahlert J (2001) Natures carbohydrate chemists: the enzymatic glycosylation of bioactive bacterial metabolites. Curr Org Chem 5(2):139–150
Weymouth-Wilson AC (1997) The role of carbohydrates in biologically active natural products. Nat Prod Rep 14(2):99–110
Yang Z, Yu BY, Zhang J, Li RM,Ge HX, Fang T, Jin P (2011a) Application of tetrahydroberberrubine in preparing anti anxiety agents and antidepressants. CN 101972252A
Yang Z, Yu BY, Zhang J, Li RM, Ge HX, Fang T, Jin P (2011b) Application of tetrahydroberberrubine in preparing medicine for treating schizophrenia. CN 102000074A
Yu BY, Ge HX, Zhu YY, Zhang J, Yang Z, Cui K (2011) 9-O-β-d-glucosyl tetrahydroberberrubine, and its preparation method and application for treating cardiovascular diseases. CN 1020022081A
Zhang WW, Yu BY, Peng J (2003) Bioconversion from l-tetrahydropalmatine to l-corydalmine. Yao Wu Sheng Wu Ji Shu 10(3): 165-168
Acknowledgment
This work was funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions and the National Nature Science Foundation of China (NSFC No. 20602040). Thanks are also given for the financial support from the State Administration of Foreign Expert Affairs of China (No. 111-2-07) and the “111 Project” from the Ministry of Education of China.
Author information
Authors and Affiliations
Corresponding author
Additional information
Hai-Xia Ge and Jian Zhang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Ge, HX., Zhang, J., Kai, C. et al. Regio- and enantio-selective glycosylation of tetrahydroprotoberberines by Gliocladium deliquescens NRRL1086 resulting in unique alkaloidal glycosides. Appl Microbiol Biotechnol 93, 2357–2364 (2012). https://doi.org/10.1007/s00253-011-3795-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-011-3795-0