Abstract
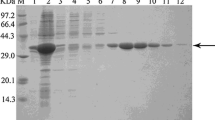
In the screening of 11 E. coli strains overexpressing recombinant oxidoreductases from Bacillus sp. ECU0013, an NADPH-dependent aldo-keto reductase (YtbE) was identified with capability of producing chiral alcohols. The protein (YtbE) was overexpressed, purified to homogeneity, and characterized of biocatalytic properties. The purified enzyme exhibited the highest activity at 50°C and optimal pH at 6.5. YtbE served as a versatile reductase showing a broad substrate spectrum towards different aromatic ketones and keto esters. Furthermore, a variety of carbonyl substrates were asymmetrically reduced by the purified enzyme with an additionally coupled NADPH regeneration system. The reduction system exhibited excellent enantioselectivity (>99% ee) in the reduction of all the aromatic ketones and high to moderate enantioselectivity in the reduction of α- and β-keto esters. Among the ketones tested, ethyl 4,4,4-trifluoroacetoacetate was found to be reduced to ethyl (R)-4,4,4-trifluoro-3-hydroxy butanoate, an important pharmaceutical intermediate, in excellent optical purity. To the best of our knowledge, this is the first report of ytbE gene-encoding recombinant aldo-keto reductase from Bacillus sp. used as biocatalyst for stereoselective reduction of carbonyl compounds. This study provides a useful guidance for further application of this enzyme in the asymmetric synthesis of chiral alcohol enantiomers.



Similar content being viewed by others
References
Bohren KM, Bullock B, Wermuth B, Gabbay KH (1989) The aldo-keto reductase superfamily. J Biol Chem 264:9547–9551
Davoli P, Forni A, Moretti I, Prati F, Torre G (1999) (R)-(+) and (S)-(−) ethyl 4, 4, 4-trifluoro-3-hydroxy butanoate by enantioselective Baker’s yeast reduction. Enzyme Microbial Tech 25:149–152
Ellis EM (2002) Microbial aldo-keto reductases. FEMS Microbiol Lett 216:123–131
Ford G, Ellis EM (2002) Characterization of Ypr1p from Saccharomyces cerevisiae as a 2-methylbutyraldehyde reductase. Yeast 19:1087–1096
Fujisawa T, Ichikawa K, Shimizu M (1993) Stereocontrolled synthesis of p-substituted trifluoromethylbenzylic alcohol derivatives of high optical purity by the baker’s yeast reduction. Tetrahedron: Asymmetry 4:1237–1240
Goldberg K, Schroer K, Lütz S, Liese A (2007) Biocatalytic ketone reduction—a powerful tool for the production of chiral alcohols—part I: processes with isolated enzymes. Appl Microbiol Biotechnol 76:237–248
Habrych M, Rodriguez S, Stewart JD (2002) Purification and identification of an Escherichia coli β-keto ester reductase as 2, 5-diketo-D-gluconate reductase YqhE. Biotechnol Prog 18:257–261
Hammond JR, Poston WB, Ghiviriga I, Feske DB (2007) Biocatalytic synthesis towards both antipodes of 3-hydroxy-3-phenylpropanitrile, a precursor to fluoxetine, atomoxetine and nisoxetine. Tetrahedron Lett 48:1217–1219
He JY, Mao XD, Sun ZH, Zheng P, Ni Y, Xu Y (2007) Microbial synthesis of ethyl (R)-4, 4, 4-trifluoro-3-hydroxybutanoate by asymmetric reduction of ethyl 4, 4, 4-trifluoroacetoacetate in an aqueous-organic solvent biphasic system. Biotechnol J 2:260–265
Hyndman D, Bauman DR, Heredia VV, Penning TM (2003) The aldo-keto reductase superfamily homepage. Chem Biol Interact 143–144:621–631
Itoh N, Asako H, Banno K, Makino Y, Shinohara M, Dairi T, Wakita R, Shimizu M (2004) Purification and characterization of NADPH-dependent aldo-keto reductase specific for β-keto esters from Penicillium citrinum, and production of methyl (S)-4-bromo-3-hydroxybutyrate. Appl Microbiol Biotechnol 66:53–62
Jez JM, Penning TM (2001) The aldo-keto reductase (AKR) superfamily: an update. Chem Biol Interact 130–132:499–525
Jez JM, Flynn TG, Penning TM (1997) A new nomenclature for the aldo-keto reductase superfamily. Biochem Pharmacol 54:639–647
Kaluzna IA, Matsuda T, Sewell AK, Stewart JD (2004) Systematic investigation of Saccharomyces cerevisiae enzymes catalyzing carbonyl reductions. J Am Chem Soc 126:12827–12832
Kaluzna IA, Feske BD, Wittayanan W, Ghiviriga I, Stewart JD (2005) Stereoselective, biocatalytic reductions of α-chloro-β-keto esters. J Org Chem 70:342–345
Kataoka M, Kita K, Wada M, Yasohara Y, Hasegawa J, Shimizu S (2003) Novel bioreduction system for the production of chiral alcohols. Appl Microbiol Biotechnol 62:437–445
Kilunga KB, Inoue T, Okano Y, Kabututu Z, Martin SK, Lazarus M, Duszenko M, Sumii Y, Kusakari Y, Matsumura H, Kai Y, SugiYama S, Inaka K, Inui T, Urade Y (2005) Structural and mutational analysis of Trypanosoma brucei prostaglandin H2 reductase provides insight into the catalytic mechanism of aldo-keto reductases. J Biol Chem 280:26371–26382
Kita K, Matsuzaki K, Hashimoto T, Yanase H, Kato N, Chung MCM, Kataoka M, Shimizu S (1996) Cloning of the aldehyde reductase gene from a red yeast, Sporobolomyces salmonicolor, and characterization of the gene and its product. Appl Environ Microbiol 62:2303–2310
Kitamura M, Ohkuma T, Takaya H, Noyori N (1988) A practical asymmetric synthesis of carnitine. Tetrahedr Lett 29:1555–1556
Kroutil W, Mang H, Edegger K, Faber K (2004) Recent advances in the biocatalytic reduction of ketones and oxidation of sec-alcohols. Curr Opin Chem Biol 8:120–126
Kunst F, Ogasawara N, Moszer I, Albertini AM, Alloni G, Azevedo V, Bertero MG, Bessieres P, Bolotin A, Borchert S, Borriss R, Boursier L, Brans A, Braun M, Brignell SC, Bron S, Brouillet S, Bruschi CV, Danchin A (1997) The complete genome sequence of the Gram-positive bacterium Bacillus subtilis. Nature 390:249–256
Lei J, Zhou YF, Li LF, Su XD (2009) Structural and biochemical analyses of YvgN and YtbE from Bacillus subtilis. Protein Sci 18:1792–1800
Matsuda T, Yamanaka R, Nakamura K (2009) Recent progress in biocatalysis for asymmetric oxidation and reduction. Tetrahedron: Asymmetry 20:513–557
Moore CJ, Pollard JD, Kosjek B, Devine NP (2007) Advances in the enzymatic reduction of ketones. Acc Chem Res 40:1412–1419
Nakamura K, Yamanaka R, Matsuda T, Harada T (2003) Recent developments in asymmetric reduction of ketones with biocatalysts. Tetrahedron: Asymmetry 14:2659–2681
Okano K, Murata K, Ikariya T (2000) Stereoselective synthesis of optically active pyridyl alcohols via asymmetric transfer hydrogenation of pyridyl ketones. Tetrahedron Lett 41:9277–9280
Petrash JM (2007) All in the family: aldose reductase and closely related aldo-keto reductases. Cell Mol Life Sci 61:737–749
Rabasseda X, Sorbera LA, Castaner J (1999) Befloxatone. Antidepressant, MAO-A inhibitor. Drugs Fut 24:1057–1067
Ramu R, Khanna GBR, Kamal A (2002) Chemoenzymatic synthesis of both enantiomers of fluoxetine, tomoxetine and nisoxetine: lipase-catalyzed resolution of 3-aryl-3-hydroxy propanenitriles. Tetrahedron: Asymmetry 13:2039–2051
Schweiger P, Gross H, Deppenmeier U (2010) Characterization of two aldo-keto reductases from Gluconobacter oxydans 621H capable of region- and stereoselective α-ketocarbonyl reduction. Appl Microbiol Biotechnol 87:1415–1426
Woodley JM (2008) New opportunities for biocatalysis: making pharmaceutical processes greener. Trends Biotechnol 26:321–327
Xie Y, Xu JH, Xu Y (2010) Isolation of a Bacillus strain producing ketone reductase with high substrate tolerance. Bioresour Technol 101:1054–1059
Yamada H, Shimizu S, Kataoka M, Sakai H, Miyoshi T (1990) A novel NADPH-dependent aldehyde reductase, catalyzing asymmetric reduction of β-keto acid esters, from Sporobolomyces salmonicolor: purification and characterization. FEMS Microbiol Lett 70:45–48
Yamamoto H, Matsuyama A, Kobayashi Y (2003) Synthesis of ethyl (S)-4-chloro-3-hydroxylbutanoate using fabG-homologues. Appl Microbiol Biotechnol 61:133–139
Zhang J, Witholt B, Li Z (2006) Efficient NADPH recycling in enantioselective bioreduction of a ketone with permeabilized cells of a microorganism containing a ketoreductase and a glucose 6-phosphate. Adv Synth Catal 348:429–433
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Nos. 20773038 and 20902023), Ministry of Science and Technology, P.R. China (Nos. 2009CB724706 and 2009ZX09501-016), China National Special Fund for State Key Laboratory of Bioreactor Engineering (No. 2060204), and Shanghai Leading Academic Discipline Project (No. B505).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Ni, Y., Li, CX., Ma, HM. et al. Biocatalytic properties of a recombinant aldo-keto reductase with broad substrate spectrum and excellent stereoselectivity. Appl Microbiol Biotechnol 89, 1111–1118 (2011). https://doi.org/10.1007/s00253-010-2941-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-010-2941-4