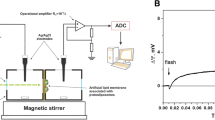
Abstract
Comparative analysis of the photoelectric response of dried films of purple membranes (PM) depending on their degree of orientation is presented. Time dependence of the photo-induced protein electric response signal (PERS) of oriented and non-oriented films to a single laser pulse in the presence of the external electric field (EEF) was experimentally determined. The signal does not appear in the non-oriented films when the EEF is absent, whereas the PERS of the oriented PM films demonstrates the variable polarity on the microsecond time scale. In the presence of the EEF the PERS of the non-oriented film rises exponentially preserving the same polarization. The polarization of the PERS changes by changing the polarity of the EEF with no influence on the time constant of the PERS kinetics. The EEF effect on the PERS of the oriented films is more complicated. By subtracting the PERS when EEF ≠ 0 from the PERS when EEF = 0 the resulting signal is comparable to that of the non-oriented films. Generalizing the experimental data we conclude that the EEF influence is of the same origin for the films of any orientation. To explain the experimental results the two-state model is suggested. It assumes that the EEF directionally changes the pKa values of the Schiff base (SB) and of the proton acceptor aspartic acid D85 in bacteriorhodopsin. Because of that the SB→D85 proton transfer might be blocked and consequently the L→M intermediate transition should vanish. Thus, on the characteristic time scale τ L → M ≈ 30 μs; both intermediates, the M intermediate, appearing under normal conditions, and the L intermediate as persisting under the blocked conditions when D85 is protonated, should coexist in the film. The total PERS is a result of the potentials corresponding to the electrogenic products of intermediates L and M that are of the opposite polarity. It is concluded that the ratio of bacteriorhodopsin concentrations corresponding to the L and M intermediates is driven by the EEF and, consequently, it should define the PERS of the non-oriented films. According to this model the orientation degree of the film could be evaluated by describing the PERS.








Similar content being viewed by others
References
Balashov SP, Govindjee R, Imasheva ES, Misra S, Ebrey TG, Feng Y, Crouch RK, Menick DR (1995) The two pKa’s of aspartate-85 and control of thermal isomerization and proton release in the arginine-82 to lysine mutant of bacteriorhodopsin. Biochemistry 34(27):8820–8834
Balashov SP, Imasheva ES, Govindjee R, Ebrey TG (1996) Titration of Aspartate-85 in bacteriorhodopsin: what it says about chromophore isomerization and proton release. biophys J 70:473–481
Bamberg E, Dencher NA, Fahr A, Heyn MP (1980) Transmembraneous incorporation of photoelectrically active bacteriorhodopsin in planar lipid bilayers. Proc Natl Acad Sci USA 78:7502–7506
Belrhali E, Nollert P, Royant D, Menzel C, Rozenbusch JR, Landau E. Pebay-Peyroula (1999) Protein, lipid and water organization in bacteriorhodopsin: a molecular view of the purple membrane at 1.9 angstroms resolution. Structure 7:909–917
Braun D, Dencher NA, Fahr A, Lindau M, Heyn MP (1988) Nonlinear voltage dependence of the light-driven proton pump current of bacteriorhodopsin. Biophys J 53:617–621
Brown LS, Dioumaev AK, Needleman R, Lanyi JK (1998) Connectivity of the retinal Sciff base to Asp85 and Asp96 during the basteriorhodopsin photocycle: the local-access model. Biophys J 75:1455–1465
Brown LS, Needleman R, Lanyi JK (2002) Conformational change of the E–F interhelical loop in the M photointermediate of bacteriorhodopsin. J Mol Biol 317:471–478
Butt HJ, Fendler K, Bamberg E, Tittor J, Oesterhelt D (1989) Aspartic acids 96 and 85 play a central role in the function of bacteriorhodopsin as a proton pump. EMBO J 8:1657–1663
Chamorovsky SK, Lukashev EP, Alexander AA, Kononenko A, Rubin AB (1983) Effects of electric field on the photocycle of bacteriorhodopsin. Biochim Biophys Acta 725:403–406
Chang CH, Chen JG, Govindjee R, Ebrey T (1985) Cation binding by bacteriorhodopsin. Proc Natl Acad Sci USA. 82:396–400
Chizmadjev YA, Aityan SK (1977) Ion transport across sodium channels in biological membranes. J Theor Biol 64(3):429–453
Chronister EL, Corcoran TC, Song L, El-Sayed MA (1986) On the molecular mechanism of the Schiff base deprotonation during the bacteriorhodopsin photocycle. Proc Natl Acad Sci USA 83:8580–8584
De Groot HJ, Smith SO, Courtin J, Van den Berg E, Winkel C, Lugtenburg J, Griffin RG, Herzfeld J (1990) Solid-state 13C and 15N NMR study of the low pH forms of bacteriorhodopsin. Biochemistry. 29:6873–6883
Dér A, Hargittai P, Simon J (1985) Time-resolved photoelectric and absorption signals from oriented purple membranes immobilized in gel. J Biochem Biophys Methods 10:295–300
Drachev LA, Kaulen AD, Skulachev VP (1978) Time resolution of the intermediate steps in the bacteriorhodopsin-linked electrogenesis. FEBS Lett 87:161–167
Edman K, Royant A, Larsson G, Jacobson F, Taylor T, van der Spoel D, Landau EM, Peyroula EP, Neutze R (2004) Deformation of helix C in the low temperature L-intermediate of bacteriorhodopsin. J Biol Chem 279:2147–2158
Essen LO, Siegert R, Lehmann WD, Oesterhelt D (1998) Lipid patches in membrane protein oligomers: crystal structure of the bacteriorhodopsin–lipid complex. Proc Natl Acad Sci USA. 95:11673–11678
Fischer U, Oesterhelt D (1979) Chromophore equilibriums in bacteriorhodopsin. Biophys J 28:211–230
Gergely C, Zimányi L, Váró G (1997) Bacteriorhodopsin intermediate spectra determined over wide pH range. J Phys Chem B 101:9390–9395
Groma GI, Kelemen L, Kulcsar A, Lakatos M, Váró G (2001) Photocycle of dried acid form of bacteriorhodopsin. Biophys J 81:3432–3441
Grudinin S, Buldt G, Gordeliy V, Baumgaertner A (2005) Water molecules and hydrogen-bonded networks in bacteriorhodopsin—molecular dynamics simulations of the ground state and the M-intermediate. Biophys J 88:3252–3261
Hellingwerf KJ, Schuurmans JJ, Westerhoff HV (1978) Demonstration of coupling between the protonmotive force across bacteriorhodopsin and the flow through its photochemical cycle. FEBS Lett 92:181–186
Hong FT, Montal M (1979) Bacteriorhodopsin in model membranes, a component of the displacement photocurrent in the microsecond time scale. Biophys J 25:465–472
Imasheva ES, Balashov SP, Ebrey TG, Chen N, Crouch RK, Menick DR (1999) Two groups control light-induced schiff base deprotonation and the proton affinity of Asp85 in the Arg82his mutant of bacteriorhodopsin. Biophys J 77:2750–2763
Kandori H (2004) Hydration switch model for the proton transfer in the Schiff base region of bacteriorhodopsin. Biochim Biophys Acta 1658:72–79
Kietis P, Vengris M, Valkunas L (2001a) Electrical-to-mechanical coupling in purple membranes: membrane as electrostrictive medium. Biophys J 80:1631–1640
Kietis P, Vengris M, Valkunas L (2001b) In: Der A, Keszthelyi L (eds) Bioelectronic applications of photochromic pigments. NATO science series, IOS Press, Amsterdam, pp 185–197
Kietis P, Linge D, Saudargas P, Valkunas L (2001c) Photoelectric response and electrostrictive properties of dried purple membrane films: the comparative study. Lith J Phys 41:477–483
Kietis PB, Saudargas P, Valkunas L (2002) Electrostriction of purple membranes and the model of active proton transfer in bacteriorhodopsin. SPIE Proc 5122:122–131
Kietis PB, Saudargas P, Valkunas L (2005) Piezoelectric model for active proton transport in bacteriorhodopsin. Lith J Phys 45:397–409
Kimura Y, Vassylyev DG, Miyazawa A, Kidera A, Matsushima M, Mitsuika K, Murata K, Hirai T, Fujiyoshi Y (1997) Surface of bacteriorhodopsin revealed by high-resolution electron crystallography. Nature 389:206–211
Kobayashi T, Ohtani H, Iwai JI, Ikegami A, Uchiki H (1983) Effect of pH on the photoreaction cycles of bacteriorhodopsin. FEBS Lett 162:197–200
Kolodner P, Lukashev EP, Ching YC, Rousseau D (1996) Electric-field-induced Schiff base deprotonation in D85N mutant bacteriorhodopsin. Proc Natl Acad Sci USA 93:11618–11621
Kolodner P, Lukashev EP, Ching YC, Rousseau D, Sheves M (1999) Electric-field effects in 13-demethyl-11,14-epoxyretinal-bacteriorhodopsin films. Photochem Photobiol 70:103–110
Kolodner P, Lukashev EP, Ching Y (2000) Electric-field effects in dry films of D85N and D8596N mutant bacteriorhodopsin. Bioelectrochemistry 51:67–73
Lanyi JK (1993) Proton translocation mechanism and energetics in the light-driven pump bacteriorhodopsin. Biochim Biophys Acta Bioenerg 1183:241–261
Lanyi JK (2000a) Molecular mechanism of ion transport in bacteriorhodopsin: insights from crystallographic, spectroscopic and mutational studies, J Phys Chem B 104:11441–11448
Lanyi JK (2000b) Bacteriorhodopsin: a special issue. Biochim Biophys Acta 1460:1–239
Lanyi JK (2004a) X-ray diffraction of bacteriorhodopsin photocycle intermediates (Review). Mol Membr Biol 21:143–150
Lanyi JK (2004b) Bacteriorhodopsin. Annu Rev Physiol 66:665–688
Lanyi JK (2004) What is real crystallographic structure of the L photointermediate of bacteriorhodopsin?. Biochim Biophys Acta 1685:14–22
Lanyi JK, Schobert B (2002) Crystallographic structure of the retinal and the protein after deprotonation of the schiff base: the switch in the bacteriorhodopsin photocycle. J Mol Biol 321:727–737
Lanyi KL, Schobert B (2003) Mechanizm of proton transport in bacteriorhodopsin from crystallographic structures of the K, L, M1, M2, and M2’ intermediates of the photocycle. J Mol Biol 328:439–450
Läuger P, Benz R, Stark G, Bamberg E, Jordan PC, Fahr A, Brock W (1981) Relaxation studies of ion transport systems in lipid bilayer membranes. Q Rev Biophys 14:513–598
Ludmann K, Gergely C, Der A, Váró G (1998) Electric signals during the bacteriorhodopsin photocycle, determined over a wide pH range. Biophys J 75:3120–3126
Luecke H, Schobert B, Richter HT, Cartailler JP, Lanyi JK (1999a) Structural changes in the M photointermediate of bacteriorhodopsin at 2 angstrom resolution. Science 286:255–260***
Luecke H, Schobert B, Richter HT, Cartailler JP, Lanyi JK (1999b) Structure of bacteriorhodopsin at 1.55 A resolution. J Mol Biol 291:899–911***
Lukashev EP, Vozary E, Kononenko AA, Rubin AB(1980) Electric field promotion of the bacteriorhodopsin BR570 to BR412 photoconversion in films of Halobacterium halobium purple membranes. Biochim Biophys Acta 592:258–266
Lukashev EP, Vozary E, Kononenko AA, Rubin AB, Abdulaev NG (1982) Electric field regulation of bacteriorhodopsin photochromic cycle (in russian). Bioorg Chem 8:1173–1179
Lukashev EP, N. Kh. Seifullina, Kolodner P (2001) Effect of the external electric field on the formation of M-product in oriented bacteriorhodopsin films (in Russian). Biol Membr 18:137–144
Mathies RA, Lin SW, Ames JB, Pollard WT (1991) From femtoseconds to biology: mechanism of bacteriorhodopsin’s light-driven proton pump, Annu Rev Biophys Biophys Chem 20:491–518
Metz G, Siebert F, Engelhard M (1992) Asp85 is the only internal aspartic acid that gets protonated in the M intermediate and the purple-to-blue transition of bacteriorhodopsin. FEBS 303:237–241
Mowery PC, Lozier RH, Chae Q, Tseng YW, Taylor M, Stoeckenius W (1979) Effect of acid pH on the absorption spectra and photoreactions of bacteriorhodopsin. Biochemistry 18:4100–4107
Neutze R, Peyroula EP, Edman K, Royant A, Navarro J, Landau EM (2002) Bacteriorhodopsin: a high-resolution structural view of vectorial proton transport. Biochim Biophys Acta 1565:144–167
Oesterhelt D, Stoeckenius W (1973) Functions of a new photoreceptor membrane. Proc Natl Acad Sci USA 70:2853–2857
Ohtani H, Kobayashi T, Iwai JI, Ikegami A (1986) Picosecond and nanosecond spectroscopies of the photochemical cycles of acidified bacteriorhodopsin. Biochemistry 25:3356–3363
Rotschild KJ (1992) FTIR difference spectroscopy of bacteriorhodopsin: toward a molecular model. J Bioenerg Biomembr 24:147–167
Rousso I, Khatchatryan E, Brodsky I, Nachustai R, Ottolenghi M, Sheves M, Lewis A (1997) Atomic force sensing of light-induced protein dynamics with microsecond time resolution in bacteriorhodopsin and photosynthetic reaction centres. J Struct Biol 119:158–164
Schobert B, Vickery JC, Hornak V, Smith SO, Lanyi JK (2002) Crystallographic structure of the K intermediate of bacteriorhodopsin: conservation of free energy after photoisomerization of the retinal. J Mol Biol 321:715–726
Simmeth R, Rayfield GW (1990) Evidence that the photoelectric response of bacteriorhodopsin occurs in less than 5 picoseconds. Biophys J 57:1099–1101
Váró G (1981) Dried oriented purple membrane samples. Acta Biol Acad Sci Hung 32:301–310
Váró G, Keszthelyi L (1983) Photoelectric signals from dried oriented purple membranes of Halobacterium halobium. Biophys J 43:47–51
Váró G, L. Keszthelyi (1985) Arrhenius parameters of the bacteriorhodopsin photocycle in dried oriented samples. Biophys J 47:243–246
Váró G, Lanyi JK (1989) Photoreactions of bacteriorhodopsin at acid pH. Biophys J 56:1143–1151
Váró G, Lanyi JK (1991) Kinetic and spectroscopic evidence for an irreversible step between deprotonation and reprotonation of the Schiff base in the bacteriorhodopsin photocycle. Biochemistry 30:5008–5015
Wang J (2000) Photocurrent from oriented membrane films containing acid-blue and acid-purple bacteriorhodopsin and its mutants. Photochem Photobiol 71(4):476–480
Zhu F, Schulten K (2003) Water and proton conduction through carbon nanotubes as models for biological channels. Biophys J 85:236–244
Acknowledgments
Authors B.P.K., P.S. and L.V. are grateful to the Lithuanian State Science and Studies Foundation and G.V. is grateful to the National Science Research Fund of Hungary OTKA T048706 for the financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kietis, B.P., Saudargas, P., Vàró, G. et al. External electric control of the proton pumping in bacteriorhodopsin. Eur Biophys J 36, 199–211 (2007). https://doi.org/10.1007/s00249-006-0120-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00249-006-0120-4