Abstract
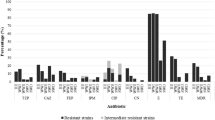
The main aim of this paper was the comprehensive estimation of the occurrence rate and the antibiotic-resistance conditions of opportunistic pathogen Pseudomonas aeruginosa in hydrocarbon-contaminated environments. From 2002 to 2007, 26 hydrocarbon-contaminated sites of Hungary were screened for the detection of environmental isolates. Altogether, 156 samples were collected and examined for the determination of appearance, representative cell counts, and antibiotic-resistance features of P. aeruginosa. The detected levels of minimal inhibitory concentrations of ten different drugs against 36 environmental strains were compared to the results of a widely used reference strain ATCC 27853 and four other clinical isolates of P. aeruginosa. Based on our long-term experiment, it can be established that species P. aeruginosa was detectable in case of 61.5% of the investigated hydrocarbon-contaminated sites and 35.2% of the examined samples that shows its widespread occurrence in polluted soil–groundwater systems. In the course of the antibiotic-resistance assay, our results determined that 11 of the examined 36 environmental strains had multiple drug-resistance against several clinically effective antimicrobial classes: cephalosporins, wide spectrum penicillins, carbapenems, fluoroquinolones, and aminoglycosides. The fact that these multiresistant strains were isolated from 8 different hydrocarbon-contaminated sites, mainly from outskirts, confirms that multiple drug-resistance of P. aeruginosa is widespread not only in clinical, but also in natural surroundings as well.


Similar content being viewed by others
References
Alonso A, Rojo F, Martínez JL (1999) Environmental and clinical isolates of Pseudomonas aeruginosa show pathogenic and biodegradative properties irrespective of their origin. Environ Microbiol 1(5):421–430
Arancibia F, Bauer TT, Ewig S, Mensa J, Gonzalez J, Niederman MS, Torres A (2002) Community-acquired pneumonia due to Gram-negative bacteria and Pseudomonas aeruginosa: incidence, risk, and prognosis. Arch Intern Med 162(16):1849–1858
Arruda EAG, Marinho IS, Boulos M, Sinto SI, Caiaffa FHH, Mendes CM, Oplustil CP, Sader H, Levy CE, Levin AS (1999) Nosocomial infections caused by multiresistant Pseudomonas aeruginosa. Infect Control Hosp Epidemiol 20(9):620–623
Atzél B, Szoboszlay S, Mikuska Zs, Kriszt B (2007) Comparison of phenotypic and genotypic methods for the detection of environmental isolates of Pseudomonas aeruginosa. Int J Hyg Environ Health 211(1–2):143–155
Babay HAH (2007) Antimicrobial resistance among clinical isolates of Pseudomonas aeruginosa from patients in a teaching hospital, Riyadh, Saudi Arabia, 2001–2005. Jpn J Infect Dis 60(2–3):123–125
Cappuccino JG, Sherman N (2008) Bacterial staining. In: Cappuccino JG, Sherman N (eds) Microbiology. A laboratory manual. Pearson Benjamin Cummings, San Francisco, pp 87–89
Chayabutra C, Ju LK (2000) Degradation of n-hexadecane and its metabolites by Pseudomonas aeruginosa under microaerobic and anaerobic denitrifying conditions. Appl Environ Microbiol 66(2):493–498
CLSI (2006) Clinical and Laboratory Standards Institute, methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; Approved Standard-Seventh Edition. M7-A7 26(2):1–64
CLSI (2007) Clinical and Laboratory Standards Institute, Performance Standards for Antimicrobial Susceptibility Testing; Seventeenth Informational Supplement. M100-S17 27(1):1–182
da Silva MEZ, Filho IC, Endo EH, Nakamura AC, Ueda-Nakamura T, Filho BDP (2008) Characterisation of potential virulence markers in Pseudomonas aeruginosa isolated from drinking water. Antonie Van Leeuwenhoek 93(4):323–334
Das K, Mukherjee AK (2007) Crude petroleum-oil biodegradation efficiency of Bacillus subtilis and Pseudomonas aeruginosa strains isolated from a petroleum-oil contaminated soil from North-East India. Bioresour Technol 98(7):1339–1345
D’Costa VM, McGrann KM, Hughes DW, Wright GD (2006) Sampling the antibiotic resistome. Science 311(5759):374–377
Draghi DC, Jones ME, Sahm DF, Tillotson GS (2006) Geographically-based evaluation of multidrug resistance trends among Streptococcus pneumoniae in the USA: findings of the FAST surveillance initiative (2003–2004). Int J Antimicrob Agents 28(6):525–531
Fu H, Zeng G, Zhong H, Yuan X, Wang W, Huang G, Li J (2007) Effects of rhamnolipid on degradation of granular organic substrate from kitchen waste by a Pseudomonas aeruginosa strain. Coll Sufr B 58(2):91–97
Gad GF, El-Domany RA, Zaki S, Ashour HM (2007) Characterization of Pseudomonas aeruginosa isolated from clinical and environmental samples in Minia, Egypt: prevalence, antibiogram and resistance mechanisms. J Antimicrob Chemother 60(5):1010–1017
Gales AC, Jones RN, Turnidge J, Rennie R, Ramphal R (2001) Characterisation of Pseudomonas aeruginosa isolates: occurrence rate, antimicrobial susceptibility pattern and molecular typing in Global SENTRY antimicrobial surveillance program 1997–1999. Clin Infect Dis 32:S146–S155
Ghazali FM, Rahman NZA, Salleh AB, Basri M (2004) Biodegradation of hydrocarbons in soil by microbial consortium. Int Biodeterior Biodegradation 54(1):61–67
Highsmith AK, Abshire RL (1975) Evaluation of a most-probable-number technique for the enumeration of Pseudomonas aeruginosa. Appl Microbiol 30(4):596–601
Hungarian Standard. MSZ 21464:1998. Sampling of groundwaters
Hungarian Standard. MSZ 21470-1:1998. Environmental protection. Testing of soils. Sampling
Hungarian Standard. MSZ 21470-77:1988. Environmental protection. Testing of soils. Microbiological test
Jarvis WR, Martone WJ (1992) Predominant pathogens in hospital infections. J Antimicrob Chemother 29(suppl A):19–24
Kalin M, Wheeler WN, Meinrath G (2004) The removal of uranium from mining waste water using algal/microbial biomass. J Environ Radioact 78(2):151–177
Kerry J, Hiney M, Coyne R, Cazabon D, NicGabhainn S, Smith P (1994) Frequency and distribution of resistance to oxytetracycline in micro-organisms isolated from marine fish farm sediments following therapeutic use of oxytetracycline. Aquaculture 123(1–2):43–44
Kümmerer K (2003) Significance of antibiotics in the environment. J Antimicrob Chemother 52(1):5–7
Libisch B, Balogh B, Füzi M (2009) Identification of two multidrug-resistant Pseudomonas aeruginosa clonal lineages with a countrywide distribution in Hungary. Curr Microbiol 58(2):111–116
Livermore DM, Hope R, Brick G, Lillie M, Reynolds R on behalf of the BSAC Working Parties on Resistance Surveillance (2008) Non-susceptibility trends among Pseudomonas aeruginosa and other non-fermentative Gram-negative bacteria from bacteraemias in the UK and Ireland, 2001–06. J Antimicrob Chemother 62(suppl 2):ii55–ii63
Madigan MT (2000) Prokaryotic diversity: bacteria. In: Madigan MT, Martinko JM, Parker J (eds) Brock biology of microorganisms. Prentice-Hall International, London, pp 453–544
Monsen T, Ronnmark M, Olofsson C, Wistrom J (1999) Antibiotic susceptibility of staphylococci isolated in blood cultures in relation to antibiotic consumption in hospital wards. Scand J Infect Dis 31(4):399–404
Moskowitz SM, Foster JM, Emerson JC, Gibson RL, Burns JL (2005) Use of Pseudomonas biofilm susceptibilities to assign simulated antibiotic regimens for cystic fibrosis airway infection. J Antimicrob Chemother 56(5):879–886
Ndip RN, Dilonga HM, Ndip LM, Akoachere JFK, Nkuo Akenji T (2005) Pseudomonas aeruginosa isolates recovered from clinical and environmental samples in Buea, Cameroon: current status on biotyping and antibiogram. Trop Med Int Health 10(1):74–81
Parveen S, Murphree RL, Edmiston L, Kaspar CW, Portier KM, Tamplin ML (1997) Association of multiple-antibiotic resistance profiles with point and nonpoint sources of Escherichia coli in Apalachicola Bay. Appl Environ Microbiol 63(7):2607–2612
Quinn JP, Rodvold KA (2000) Antibiotic policies in neonatal intensive-care units. Lancet 355(9208):946–947
Rice LB (2006) Challenges in identifying new antimicrobial agents effective for treating infections with Acinetobacter baumannii and Pseudomonas aeruginosa. Clin Infect Dis 43(suppl 2):S100–S105
Ridgway HF, Safarik J, Phipps D, Carl P, Clark D (1990) Identification and catabolic activity of well-derived gasoline-degrading bacteria from a contaminated aquifer. Appl Environ Microbiol 56(11):3565–3575
Robertson BK, Jjemba PK (2005) Enhanced bioavailability of sorbed 2, 4, 6-trinitrotoluene (TNT) by a bacterial consortium. Chemosphere 58(3):263–270
Sader HS, Gales AC, Pfaller MA, Mendes RE, Zoccoli C, Barth A, Jones RN (2001) Pathogen frequency and resistance patterns in Brazilian hospitals: summary of results from three years of the SENTRY antimicrobial surveillance program. Braz J Infect Dis 5(4):200–214
Saul DJ, Aislabie JM, Brown CE, Harris L, Foght JM (2005) Hydrocarbon contamination changes the bacterial diversity of soil from around Scott Base, Antarctica. FEMS Microbiol Ecol 53(1):141–155
Spilker T, Coenye T, Vandamme P, LiPuma JJ (2004) PCR-based assay for differentiation of Pseudomonas aeruginosa from other Pseudomonas species recovered from cystic fibrosis patients. J Clin Microbiol 42(5):2074–2079
SSC (1999) Opinion of the Scientific Steering Committee on Antimicrobial Resistance. European Commission. Directorate-General XXIV; 1999. Available at: http://europa.eu.int/comm/food/fs/sc/ssc/out50_en.html
Trypathy S, Kumar N, Monathy S, Samanta M, Mandal RN, Maiti NK (2007) Characterisation of Pseudomonas aeruginosa isolated from freshwater culture systems. Microbiol Res 162(4):391–396
Wilson R, Dowling RB (1998) Pseudomonas aeruginosa and other related species. Thorax 53(3):213–219
Wróblewska M (2006) Novel therapies of multidrug-resistant Pseudomonas aeruginosa and Acinetobacter spp. infections: the state of the art. Arch Immunol Ther Exp 54(2):113–120
Zavascki AP, Barth AL, Fernandes JF, Moro AL, Gonçalves AL, Goldani LZ (2006) Reappraisal of Pseudomonas aeruginosa hospital acquired pneumonia mortality in the era of metallo-β-lactamase-mediated multidrug resistance: a prospective observational study. Crit Care 10(4):R11
Acknowledgments
This research work was supported by the Ányos Jedlik Programme (OM 00120/2007) and Regional University Center of Excellence in Environmental Industry Based on Natural Resources, Szent István University (PÁZMÁNY, RET-12/2005).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kaszab, E., Kriszt, B., Atzél, B. et al. The Occurrence of Multidrug-Resistant Pseudomonas aeruginosa on Hydrocarbon-Contaminated Sites. Microb Ecol 59, 37–45 (2010). https://doi.org/10.1007/s00248-009-9551-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-009-9551-7