Abstract
Tuberculosis (TB) remains a global health problem and is the second leading cause of death from a single infectious agent, behind the novel coronavirus disease of 2019. Children are amongst the most vulnerable groups affected by TB, and imaging manifestations are different in children when compared to adults. TB primarily involves the lungs and mediastinal lymph nodes. Clinical history, physical examination, laboratory examinations and various medical imaging tools are combined to establish the diagnosis. Even though chest radiography is the accepted initial radiological imaging modality for the evaluation of children with TB, this paper, the first of two parts, aims to discuss the advantages and limitations of the various medical imaging modalities and to provide recommendations on which is most appropriate for the initial diagnosis and assessment of possible complications of pulmonary TB in children. Practical, evidence-based imaging algorithms are also presented.
Graphical Abstract

Similar content being viewed by others
Introduction
Tuberculosis (TB) remains a global health concern, particularly in Asia, Africa, Latin America and Eastern Europe. Despite the advances in diagnosis and treatment protocol, the worldwide TB burden remains enormous. Tuberculosis was the leading cause of death from a single infectious agent until the emergence of the severe acute respiratory syndrome coronavirus 2 infection, coronavirus disease 2019 [1].
Children are amongst the most vulnerable groups because of their immature immune system and other related factors. Radiological manifestations of TB in children differ from those in adults, with pulmonary disease and lymphadenopathy being the most common thoracic TB manifestations in children. These abnormalities demonstrate a spectrum of both clinical and imaging manifestations.
Even with technological improvements in the armamentarium of medical imaging tools, chest radiography (CXR) is still the radiological standard in the initial evaluation of children with suspected TB. Medical imaging is also helpful in the assessment of acute and chronic complications, response to treatment, for image-guided intervention and in excluding other underlying pathologic processes. The purposes of this article are to (1) discuss the various imaging modalities utilized in the evaluation of suspected thoracic TB in children; (2) highlight the strengths and limitations of these imaging modalities in the diagnosis of pediatric thoracic TB; (3) review the spectrum of both clinical and imaging manifestations of thoracic TB; (4) provide a practical approach and recommendations for the diagnosis of thoracic TB in children through imaging; and (5) present an up-to-date evidence-based imaging algorithm that will serve as a guide for both radiologists and clinicians.
An extensive search of the medical literature, which included peer-reviewed systematic reviews and meta-analyses, review articles, evidence-based guidelines and consensus statements, was performed. All the available information was synthesized, considering the available clinical scenarios and spectrum of thoracic TB manifestations, as well as availability of resources (which affects the choice of imaging). The authors are well-published, practice in TB endemic areas and have an average of more than 21 years’ experience (range from 16 years to 24 years). Most (S.A., N.D.P.C., B.F.L. and K.S.S.) are also members of the World Federation of Pediatric Imaging (WFPI) childhood tuberculosis group.
Pulmonary tuberculosis
Overview
Tuberculosis can affect virtually all organs of the body, but most commonly involves the thorax [2, 3]. Thoracic involvement in childhood TB is primarily nodal, pulmonary or a combination of these [4].
In children, diagnosis is usually epidemiological and indirect. Symptoms which are nonspecific include but are not limited to chronic cough, weight loss or failure to thrive, persistent unexplained fever and/or persistent unexplained lethargy or reduced activity. Childhood TB can be classically categorized as TB exposure, TB infection, possible or probable TB disease or confirmed TB disease depending on various factors: (1) presence of exposure, (2) reaction to the tuberculin skin test or interferon-gamma release assay, (3) presence or absence of symptoms, (4) imaging findings and/or (5) result of TB culture, Xpert Mycobacterium tuberculosis/rifampicin assay or acid-fast bacilli test if available [5]. Moreover, TB disease may be stratified into non-severe, severe and advanced/extensive disease, depending on symptomatology and imaging findings [6].
Imaging plays a major role in the diagnosis and follow-up (to assess treatment response and for detection of complications) in thoracic TB. In general, particularly in low-resource areas, the main diagnostic imaging tools are CXR and/or ultrasound (US) where available, while computed tomography (CT) and magnetic resonance imaging (MRI) which are mainly available in high-resource areas or tertiary centers are very costly and reserved for complicated cases [4, 5, 7, 8].
Imaging modalities
Chest radiography
The CXR remains an important imaging tool for patients suspected to have pulmonary TB [4, 5, 9,10,11,12]. It is however insensitive and nonspecific for TB and has poor interobserver agreement [5, 10, 13,14,15,16,17,18,19]. Up to 15% of patients with confirmed TB may have normal CXR findings [4, 5, 20,21,22]. The use of CXR as a screening tool in pediatric TB contacts is strongly supported, except for completely asymptomatic subjects in low-resource settings. Although the frequency of abnormal CXR findings that are consistent with TB amongst asymptomatic pediatric contacts is very low [23], some recent studies show that radiographic findings inconsistent with TB in these children may indicate beginning or subclinical disease [24, 25]. Pulmonary TB has been shown to be more common (P < 0.05) in children with co-morbidities than in those without [26].
Regional (hilar or mediastinal) lymphadenopathy is the radiological hallmark (but not pathognomonic) of primary TB disease in childhood [4, 5, 11, 13, 16, 27,28,29,30], with or without lung parenchymal opacities [11, 30]. Lymphadenopathy can be visualized in 50% to 70% of cases from 1–3 months after exposure [5, 8], and decreases with increasing age [13, 14]. The prevalence of lymphadenopathy approaches 100% in children below 3 years of age but is lower (88%) in older children. In contrast, the prevalence of parenchymal involvement is lower in children less than 3 years of age (51%) and significantly higher (78%) in older children [5, 28]. However, it is difficult to identify with certainty the presence of enlarged lymph nodes on CXR [5, 19, 31,32,33], because lymphadenopathy in young children can be obscured by vascular and other mediastinal structures and particularly by a large thymus [10, 12, 19, 31, 33].
In children with inadequate immunity, the primary disease can progress contiguously or hematogenously [5, 34]. Contiguous progression of disease is seen as homogeneous parenchymal consolidation and lymphadenopathy. Compression of an airway by adjacent enlarged nodes results in overinflation (if partial obstruction) or atelectasis (if complete obstruction) because of smaller and more pliable airways in children [5, 11, 13, 14, 30, 35, 36]. The most reliable radiographic feature of lymphadenopathy is airway compression [13, 37] (Fig. 1). This lymphobronchial involvement/airway compression is strongly associated with confirmed pulmonary TB, significantly more frequently observed in infants compared to older children, although limited again by poor interobserver agreement [11, 12, 31, 33, 38]. Further imaging with cross-sectional modalities is thus recommended.
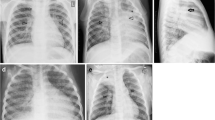
A 12-year-old boy with severe pulmonary tuberculosis with lymphobronchial involvement. a, b Posteroanterior (a) and lateral (b) radiographs of the chest show lobulated soft tissue densities in the hilar and mediastinal regions (arrowheads) representing enlarged lymph nodes, with narrowing of the left main bronchus (thick arrow) and leftward displacement of the trachea (thin arrow). c, d Contrast enhanced computed tomgraphy scan of the chest. The axial image on soft tissue window setting (c) demonstrates the typical central necrosis with a peripheral rim of enhancement in the subcarinal lymph nodes (arrowheads). The coronal image on lung window setting (d) better delineates the smooth narrowing of the left main bronchus (arrow) caused by the enlarged nodes
Hematogenously disseminated infection is termed “miliary TB” and presents with innumerable ≤ 2 mm non-calcified nodules diffusely scattered throughout both lungs [5, 8, 32, 35, 39, 40]. This nodular pattern, although not 100% specific, is commonly seen in TB [5, 40]. However, the miliary pattern can be easily missed on the CXR in up to 50% of cases [41, 42] and CXR is initially normal in 25% to 40% of cases [5, 8, 13, 32,33,34,35,36,37,38,39,40,41,42,43].
Pleural involvement is a common complication of thoracic TB and its prevalence increases with age. Pleural effusions can be associated with air-space consolidation and may be bilateral or loculated [5].
Congenital TB is an extremely rare Mycobacterium tuberculosis infection transmitted from the mother to the fetus [44] with only 300–400 reported cases [45, 46]. Chest radiographs may be normal initially [44,45,46,47,48]. The most common reported findings which may appear after 4–8 weeks are miliary pattern, multiple pulmonary nodules, lobar pneumonia and rarely, bronchopneumonia, interstitial pneumonia and mediastinal lymphadenopathy [45, 46, 48]. Ultrasound may show hypoechoic foci in the liver or spleen, ascites and abdominal lymphadenopathy. CT and MRI, if available, may be useful for unconfirmed or complicated cases [8, 46].
A CXR provides supportive evidence for diagnosing a patient suspected to have extra-pulmonary TB; e.g., 20% to 60% of children with abdominal TB have pulmonary disease [49, 50].
Ultrasound
Ultrasonography has many advantages over other imaging modalities, namely, (1) no ionizing radiation, (2) no sedation needed, (3) more cost-effective than cross-sectional imaging, and (4) can be portable. Because of these, US is becoming universally available even in low-resource areas [4, 10, 16, 51].
Ultrasound is primarily used in the evaluation of pleural fluid, but recent technological advancements have also improved the spatial resolution, tissue penetration and characterization of the peripheral lung parenchyma, and US may be useful for the detection of mediastinal lymphadenopathy [14, 51,52,53,54,55,56].
Mediastinal US, particularly in low-resource settings, may be better than CXR for the detection of enlarged lymph nodes [4, 10, 57], although it is limited by operator dependence [4, 13, 30]. Up to 67% of mediastinal lymphadenopathy was identified on US in children with pulmonary TB who had normal CXR [4, 11, 16, 58]. Interrater agreement was moderate for US (0.56) compared to CXR (0.27) [59, 60] and there was more than 80% agreement between US and CT [11, 58, 59]. Scanning of the mediastinum is performed via a suprasternal approach to visualize the paratracheal and aortopulmonary regions and via an intercostal left parasternal approach to evaluate the subcarinal and prevascular regions [4, 10, 11, 52, 57, 60].
A recent systematic review shows that detection of mediastinal lymphadenopathy with US ranges from 15% to 85%, likely due to differences in study methodology [11, 52, 57, 59]. Although there are no clear criteria for the definition of TB lymphadenopathy [59, 61], there is a statistically significant association of larger lymph nodes with pulmonary TB compared to other respiratory infections [52, 59]. Lymph nodes appear on US as well-defined, ovoid or round hypoechoic structures in comparison to anechoic elongated blood vessels [4, 10, 11, 13, 52], easily confirmed by color Doppler US [10].
Although resolution of tuberculous mediastinal lymphadenopathy is slower than mediastinal non-tuberculous lymphadenopathy, more research is needed to better understand this dynamic [59]. The noninvasive nature of US in the evaluation of mediastinal lymphadenopathy makes this a valuable tool for the follow-up of children undergoing TB treatment [11, 13, 14, 56].
Ultrasound also has significantly higher interrater agreement than CXR for pleural effusion and it can differentiate consolidation from pleural effusion [60]. Although pleural effusion is not diagnostic of pulmonary TB, pleural effusion is more common in children with pulmonary TB [52] and is often associated with consolidation [60].
Computed tomography
CT is a very useful imaging modality in the evaluation of the lungs, mediastinum and chest wall in infants and children. Compared to CXR where there is superimposition of structures, cross-sectional images are best suited to visualize the pattern and extent of parenchymal disease and lymphadenopathy [13, 51, 56]. However, CT may be inaccessible in a low-resource setting where TB is highly prevalent, and entails not only radiation exposure [5, 8, 10, 19], but also intravenous contrast injection and some immobilization. Multi-detector, dual-energy and the emerging photon-counting detector CT technologies are now available not only for better image quality and increasing lesion detection, but also decreasing the radiation dose and sedation rates, which are important advantages especially for infants and young children [51, 62].
CT is the gold standard for the detection of lymphadenopathy and its complications [10, 14, 19, 30, 31, 33, 63, 64] such as airway compression by lymphadenopathy, termed “lymphobronchial TB” (Fig. 1). Presence, pattern (smooth or irregular) and degree of tracheal and bronchial compression causing segmental or lobar collapse or overinflation, bronchiectasis and obstructive pneumonia can be determined by CT [4, 5, 13, 19, 36, 38].
Children with lymphobronchial TB may initially manifest with unremitting cough, stridor and wheezing which may reflect partial airway obstruction. CT can assess this group of patients not only by detecting lymphadenopathy, but also by evaluating and staging associated airway compression and downstream lung parenchymal disease, thereby aiding clinicians to decide the course of treatment [13, 38, 63].
The staging of post-obstructive lung disease differentiates reversible disease from irreversible lung destruction and requires the administration of intravenous contrast. This staging and progression of disease depends on the degree of airway obstruction, whether partial or complete and presence of nodal erosion into the airway lumen. Reversible lung injury is treated conservatively, and includes air trapping, consolidation showing air bronchogram with or without atelectasis and consolidation with fluid bronchogram (“drowned lung”) with or without expansile pneumonia. Non-enhancement and cavitation are features of an irreversible and non-salvageable lung which is managed with lobectomy [13, 63].
Pleural effusion can evolve into an exudative effusion or empyema [5, 8] seen on contrast-enhanced CT scan as smooth thickening and enhancement of the visceral and parietal pleura (“split-pleura” sign). An air-fluid level in the pleural cavity may indicate the presence of a bronchopleural fistula. The infected fluid can also extend beyond the parietal pleura to become a subcutaneous abscess, also known as empyema necessitans [5] [Fig. 2].
Magnetic resonance imaging
MRI has emerged as an alternative to CT for imaging children with pulmonary infections and immunocompromised patients [4, 65]. MRI is an imaging modality that has no ionizing radiation, which makes it an attractive alternative. It may be used for diagnosis and follow-up in older cooperative children and patients allergic to iodinated contrast media [66, 67]. Gadolinium-based intravenous contrast media may be administered but are not required [4]. However, MRI has disadvantages; it is less sensitive than CT in the evaluation of the lung parenchyma [51, 68,69,70], scan times are longer, often necessitating sedation in infants and young children below 6 years old and in patients with claustrophobia and MRI is expensive and not widely available in low-resource settings [4, 14, 19, 51, 63, 71].
With technological advances to reduce motion artifact and new protocols for faster acquisition times, MRI of the lung can be used in various clinical applications [4, 7, 51, 70]. MRI is comparable to CT in childhood TB for detecting mediastinal lymph nodes greater than 7 mm in size with sensitivity, specificity and positive and negative predictive values of 100%, as well as in the detection of pulmonary consolidation, nodules greater than 3 mm, presence of cysts or cavities and pleural effusions [14, 56, 66, 70]. MRI is also more sensitive when compared to unenhanced CT for nodal and pleural disease in pulmonary TB [4, 14, 66, 67]. Interobserver agreement ranges from near perfect to perfect in the detection of consolidation, pleural effusion, cyst/cavity, lymph nodes, hyperinflation and bronchiectasis [4]. However, ground glass opacities, small nodules and calcified nodules may be missed [56, 71].
Another advantage of MRI is that it can determine disease activity within the infected nodes by the presence of restricted diffusion and post-contrast enhancement [4, 13, 14]. MRI can differentiate TB lymphadenopathy from reactive lymph nodes using short tau inversion recovery/T2-weighted sequences which can demonstrate the characteristic low signal in TB [13, 14] due to the presence of paramagnetic free radicals secreted from active phagocytic cells [72]. In contrast, central hyperintensity indicates liquefactive necrosis [4, 19, 73].
For pleural effusion or empyema, MRI is as sensitive and specific as CT, and according to Sodhi and colleagues, MRI is better than CT in demonstrating septations and internal debris as well as paraspinal soft tissue and chest wall muscle involvement [66, 74]. Diffusion restriction of the fluid collection may help differentiate empyema and abscess from transudative pleural effusion.
MRI, unlike CT, can differentiate caseating TB consolidation from non-caseating consolidation secondary to other causes. The former characteristically has low T2 signal intensity [4, 14, 56, 63, 73], while the latter has high T2 signal intensity [63].
To minimize radiation exposure, MRI is appropriate in the follow-up of nodal and parenchymal disease in children with thoracic TB [4].
Positron emission tomography
Positron emission tomography (PET) is commonly used in the imaging of cancers but less utilized for infectious diseases. PET using 18F-fluorodeoxyglucose (FDG) is said to be highly sensitive for detecting tuberculous lesions [75,76,77,78]. FDG-PET/CT scan also assesses disease activity and monitors response to therapy. Active tuberculomas are FDG-avid (maximum standardised uptake value [SUVmax ]> 2.5) while inactive disease has an SUVmax < 1.5) [78]. However, differentiation between oncological, inflammatory and infectious processes is challenging [77]. The lymphadenopathy of active TB is also FDG-avid but is difficult to distinguish from other causes of lymphadenopathy, such as lymphoma [78]. Pathogen-specific imaging approaches using compounds such as radioanalogues of para-aminobenzoic acid [77, 79] and trehalose [77, 80] are still in the early stages of development but in the future could improve the sensitivity of PET. Further studies are recommended to assess the potential role of PET in the diagnosis of thoracic TB in children.
The recommended imaging modalities and algorithm for children with suspected pulmonary TB are summarized in Table 1 and Fig. 3, respectively.
Evidence-based algorithm for the imaging of children with suspected pulmonary tuberculosis (TB). a not all criteria are present in all cases. In certain cases of TB disease: (1) history of TB exposure may be difficult to establish. (2) There may be false negative TST/IGRA. (3) There may be false negative CXR. (4) There may be false negative culture/Xpert/AFB due to the paucibacillary nature of TB in children. AFB acid-fast bacilli, CT computed tomography, CXR chest X-ray, IGRA interferon-gamma release assay, IV intravenous, MRI magnetic resonance imaging, TST tuberculin skin test, US ultrasound, (+) positive or present, (-) negative or absent
Conclusion
Tuberculosis primarily affects the lungs and lymph nodes but can have a diverse spectrum of thoracic manifestations and complications. Medical imaging, along with clinical data and laboratory examinations, plays a crucial role in the diagnosis, characterization and documentation of the extent of the affected structures and in the evaluation of complications in children suspected of having TB. Knowledge of the appropriate imaging tool for a specific clinical indication is important. This will depend on the severity of the patient’s condition, age, availability of imaging resources and expertise of the interpreting radiologist or physician. Imaging recommendations and algorithms are presented in this article which are intended to help guide both radiologists and clinicians in making timely and accurate medical decisions.
References
Global tuberculosis report (2022). Geneva: World Health Organization. Licence: CC BY-NC-SA 3.0 IGO. https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2022. Accessed 26 February 2023
Baykan AH, Sayiner HS, Aydin E et al (2022) Extrapulmonary tuberculosıs: an old but resurgent problem. Insights Imaging 13:39
Kritsaneepaiboon S, Andres MM, Concepcion NDP et al (2017) Extrapulmonary involvement in pediatric tuberculosis. Pediatr Radiol 47:1249–1259
Sodhi KS, Bhalla AS, Mahomed N, Laya BF (2017) Imaging of thoracic tuberculosis in children: current and future directions. Pediatr Radiol 47:1260–1268
Concepcion NDP, Laya BF, Andronikou S et al (2017) Standardized radiographic interpretation of thoracic tuberculosis in children. Pediatr Radiol 47:1237–1248
WHO consolidated guidelines on tuberculosis (2022). Module 5: management of tuberculosis in children and adolescents. Geneva: World Health Organization. Licence: CC BY-NC-SA 3.0 IGO. https://www.who.int/publications/i/item/9789240046764. Accessed October 20, 2022
Ryu YJ (2015) Diagnosis of pulmonary tuberculosis: recent advances and diagnostic algorithms. Tuberc Respir Dis 78:64–71
Perez-Velez CM, Marais BJ (2012) Tuberculosis in children. N Engl J Med 367:348–361
Dawani A, Gupta AK, Jana M (2019) Imaging in pediatric extra-pulmonary tuberculosis. Indian J Pediatr 86:459–467
Pool KL, Heuvelings CC, Bélard S et al (2017) Technical aspects of mediastinal ultrasound for pediatric pulmonary tuberculosis. Pediatr Radiol 47:1839–1848
Bosch-Marcet J, Serres-Cre’ixams X, Zuasnabar-Cotro A et al (2004) Comparison of ultrasound with plain radiography and CT for the detection of mediastinal lymphadenopathy in children with tuberculosis. Pediatr Radiol 34:895–900
Richter-Joubert L, Andronikou S, Workman L et al (2017) Assessment of airway compression on chest radiographs in children with pulmonary tuberculosis. Pediatr Radiol 47:1283–1291
Nel M, Franckling-Smith Z, Pillay T et al (2022) Chest imaging for pulmonary TB-an update. Pathogens 11:161
Pillay T, Andronikou S, Zar HJ (2020) Chest imaging in paediatric pulmonary TB. Paediatr Respir Rev 36:65–72
Zar HJ, Moore DP, Andronikou S et al (2020) Diagnosis and management of community-acquired pneumonia in children: South African Thoracic Society guidelines. South Afr Med J 110:588–593
Bélard S, Andronikou S, Pillay T et al (2014) New imaging approaches for improving diagnosis of childhood tuberculosis. S Afr Med J 104:181–182
Dawson R, Masuka P, Edwards DJ et al (2010) Chest radiograph reading and recording system: evaluation for tuberculosis screening in patients with advanced HIV. Int J Tuberc Lung Dis 14:52–58
Zar HJ (2007) Diagnosis of pulmonary tuberculosis in children – what’s new? S Afr Med J 97:983–985
Andronikou S, Wieselthaler N (2004) Modern imaging of tuberculosis in children: thoracic, central venous system and abdominal tuberculosis. Pediatr Radiol 34:861–875
Restrepo CS, Katre R, Mumbower A (2016) Imaging manifestations of thoracic tuberculosis. Radiol Clin N Am 54:453–473
Harisinghani MG, McLoud TC, Shepard JA et al (2000) Tuberculosis from head to toe. Radiographics 20:449–470
Piccazzo R, Paparo F, Garlaschi G (2014) Diagnostic accuracy of chest radiography for the diagnosis of tuberculosis (TB) and its role in the detection of latent TB infection: a systematic review. J Rheumatol Suppl 91:32–40
Triasih R, Robertson C, de Campo J et al (2015) An evaluation of chest X-ray in the context of community-based screening of child tuberculosis contacts. Int J Tuberc Lung Dis 19:1428–1434
Huang C-C, Tan Q, Becerra MC et al (2022) The contribution of chest radiography to the clinical management of children exposed to tuberculosis. Am J Respir Crit Care Med 206:892–900
Marais BJ, Graham SM (2022) The value of chest radiography in tuberculosis preventive treatment screening in children and adolescents. Am J Respir Crit Care Med 206:814–816
Piskur ZI, Mykolyshyn LI (2021) Comorbidities at the tuberculosis among children. Wiad Lek. 74:2433–2438
Omlor GJ (2001) Pulmonary lymphadenopathy. Pediatr Infect Dis 20:437–438
Leung AN, Muller NL, Pineda PR et al (1992) Primary tuberculosis in childhood: radiographic manifestations. Radiology 182:87–91
Kim WS, Choi J II, Kim I-O et al (2006) Pulmonary tuberculosis in infants: radiographic and CT findings. AJR Am J Roentgenol 187:1024–1033
Jaganath D, Beaudry J, Salazar-Austin N (2022) Tuberculosis in Children. Infect Dis Clin North Am 36:49–71
George A, Andronikou S, Pillay T et al (2017) Intrathoracic tuberculous lymphadenopathy in children: a guide to chest radiography. Pediatr Radiol 47:1277–1282
Marais BJ, Gie RP, Simon Schaaf H et al (2004) A proposed radiological classification of childhood intra-thoracic tuberculosis. Pediatr Radiol 34:886–894
Swingler GH, du Toit G, Andronikou S et al (2005) Diagnostic accuracy of chest radiography in detecting mediastinal lymphadenopathy in suspected pulmonary tuberculosis. Arch Dis Child 90:1153–1156
Leung AN (1999) Pulmonary tuberculosis: the essentials. Radiology 210:307–322
Tomá P, Lancella L, Menchini L et al (2017) Radiological patterns of childhood thoracic tuberculosis in a developed country: a single institution’s experience on 217/255 cases. Radiol Med 122:22–34
Laya BF, Concepcion NDP, Dela Eva RC et al (2011) Computed tomography and fiberoptic bronchoscopy correlation of central airways involvement in children with primary progressive tuberculosis. St Luke’s J Med 7:11–20
Andronikou S, Vanhoenacker FM, De Backer AI (2009) Advances in imaging chest tuberculosis: Blurring of differences between children and adults. Clin Chest Med 30:717–744
Lucas S, Andronikou S, Goussard P et al (2012) CT features of lymphobronchial tuberculosis in children, including complications and associated abnormalities. Pediatr Radiol 42:923–931
Van Dyck J, Vanhoenacker FM, Van den Brande P et al (2003) Imaging of pulmonary tuberculosis. Eur Radiol 13:1771–1785
Pulido KGC, Laya BF, Villamor CP et al (2011) Radiographic patterns of active pulmonary tuberculosis disease in Filipino pediatric patients and their correlation with symptoms, length of hospital confinement and clinical dispositions. St Luke’s J Med 7:21–32
Sharma SK, Mohan A, Sharma A (2012) Challenges in the diagnosis & treatment of miliary tuberculosis. Indian J Med Res 135:703–730
Lee KS, Kim TS, Han J et al (1999) Diffuse micronodular lung disease: HRCT and pathologic findings. J Comput Assist Tomogr 23:99–106
Kwong JS, Carignan S, Kang EY et al (1996) Miliary tuberculosis: diagnostic accuracy of chest radiography. Chest 110:339–342
Daltro P, Nunez-Santos E, Laya BF (2014) Pediatric tuberculosis. In: García-Peña P, Guillerman RP (eds) Pediatric Chest Imaging, 3rd edn. Springer Verlag Berlin Heidelberg, p 297
Peng W, Yang J, Liu E (2011) Analysis of 170 cases of congenital TB reported in the literature between 1946 and 2009. Pediatr Pulmonol 46:1215–1224
Newberry DM, Bell TR (2018) Congenital tuberculosis – a new concern in the neonatal intensive care unit. Adv Neonatal Care 18:341–349
Schaaf HS, Collins A, Bekker A, Davies PDO (2010) Tuberculosis at extremes of age. Respirology 15:747–763
Shao Y, Hageman JR, Shulman ST (2021) Congenital and perinatal tuberculosis. Neoreviews 22:e600–e605
Sartoris G, Seddon JA, Rabie H et al (2020) Abdominal tuberculosis in children: challenges, uncertainty, and confusion. J Pediatric Infect Dis Soc 9:218–227
Zhao J, Cui MY, Chan T et al (2015) Evaluation of intestinal tuberculosis by multi-slice computed tomography enterography. BMC Infect Dis 15:577
Laya BF, Concepcion NDP, Garcia-Peña P et al (2022) Pediatric lower respiratory tract infections - imaging guidelines and recommendations. Radiol Clin N Am 60:15–40
Heuvelings CC, Bélard S, Andronikou S (2019) Chest ultrasound findings in children with suspected pulmonary tuberculosis. Pediatr Pulmonol 54:463–470
Coley BD (2011) Chest sonography in children: current indications, techniques, and imaging findings. Radiol Clin North Am 49:825–846
Goh Y, Kapur J (2016) Sonography of the pediatric chest. J Ultrasound Med 35:1067–1080
Joshi P, Vasishta A, Gupta M (2019) Ultrasound of the pediatric chest. Br J Radiol 92:20190058
Tonne EO, Fosbøl MØ, Poulsen A et al (2023) Imaging modalities for pulmonary tuberculosis in children: a systematic review. Eur J Radiol Open 10:100472
Moseme T, Andronikou S (2014) Through the eye of the suprasternal notch: point-of-care sonography for tuberculous mediastinal lymphadenopathy in children. Pediatr Radiol 44:681–684
Bosch-Marcet J et al (2007) Value of sonography for follow-up of mediastinal lymphadenopathy in children with tuberculosis. J Clin Ultrasound 35:118–124
Ruby LC, Heuvelings CC, Grobuch MP et al (2022) Transthoracic mediastinal ultrasound in childhood tuberculosis: a review. Paediatr Respir Rev 41:40–48
Heuvelings CC, Bélard S, Andronikou S (2019) Chest ultrasound compared to chest X-ray for pediatric pulmonary tuberculosis. Pediatr Pulmonol 54:1914–1920
Andronikou S (2002) Pathological correlation of CT-detected mediastinal lymphadenopathy in children: the lack of size threshold criteria for abnormality. Pediatr Radiol 32:912
Esquivel A, Ferrero A, Mileto A (2022) Photon-counting detector CT: key points radiologists should know. Korean J Radiol 23:854–865
Andronikou S, Lucas S, Zouvani A, Goussard P (2021) A proposed CT classification of progressive lung parenchymal injury complicating pediatric lymphobronchial tuberculosis: from reversible to irreversible lung injury. Pediatr Pulmonol 56:3657–3663
Andronikou S, Brauer B, Galpin J et al (2005) Interobserver variability in the detection of mediastinal and hilar lymph nodes on CT in children with suspected pulmonary tuberculosis. Pediatr Radiol 35:425–428
Chung JH, Huitt G, Yagihashi K et al (2016) Proton magnetic resonance imaging for initial assessment of isolated Mycobacterium avium complex pneumonia. Ann Am Thor Soc 13:49–57
Sodhi KS, Sharma M, Saxena AK et al (2017) MRI in thoracic tuberculosis of children. Indian J Pediatr 84:670–676
Rizzi EB, Schinina’ V, Cristofaro M et al (2011) Detection of pulmonary tuberculosis: comparing MR imaging with HRCT. BMC Infect Dis 11:243
Wielpütz M, Kauczor HU (2012) MRI of the lung: state of the art. Diagn Interv Radiol 18:344–353
Hirsch W, Sorgea I, Krohmera S et al (2018) MRI of the lungs in children. Eur J Radiol 68:278–288
Sodhi KS, Khandelwal N, Saxena AK et al (2016) Rapid lung MRI in children with pulmonary infections: time to change our diagnostic algorithms. J Magn Reson Imaging 43:1196–1206
Sodhi KS, Ciet P, Vasanawala S (2022) Practical protocol for lung magnetic resonance imaging and common clinical indications. Pediatr Radiol 52:295–311
Joshi AR, Basantani AS (2014) Role of CT and MRI in abdominal tuberculosis. Curr Radiol Rep 2:66
Peprah KO, Andronikou S, Goussard P (2012) Characteristic magnetic resonance imaging low T2 signal intensity of necrotic lung parenchyma in children with pulmonary tuberculosis. J Thorac Imaging 27:171–174
Sodhi KS, Bhatia A, Nichat V et al (2021) Chest MRI as an emerging modality in the evaluation of empyema in children with specific indications: pilot study. Pediatr Pulmonol 56:2668–2675
Chen RY, Dodd LE, Lee M et al (2014) PET/CT imaging correlates with treatment outcome in patients with multidrug-resistant tuberculosis. Sci Transl Med 6:265ra166
Ankrah AO, van der Werf TS, de Vries EF et al (2016) PET/CT imaging of Mycobacterium tuberculosis infection. Clin Transl Imaging 4:131–144
Jain SK, Andronikou S, Goussard P et al (2020) Advanced imaging tools for childhood tuberculosis: potential applications and research needs. Lancet Infect Dis 20:e289–e297
Pelletier-Galarneau M, Martineau P, Zuckier LS et al (2017) 18F-FDG-PET/CT imaging of thoracic and extrathoracic tuberculosis in children. Semin Nucl Med 47:304–318
Ordonez AA, Weinstein EA, Bambarger LE et al (2017) A systematic approach for developing bacteria-specific imaging tracers. J Nucl Med 58:144–150
Peña-Zalbidea S, Huang AY, Kavunja HW et al (2019) Chemoenzymatic radiosynthesis of 2-deoxy-2-[18F]fluoro-d-trehalose ([18F]-2-FDTre): a PET radioprobe for in vivo tracing of trehalose metabolism. Carbohydr Res 472:16–22
Author information
Authors and Affiliations
Contributions
B.F.L., N.D.P.C., S.A., Z.A.M., M.I.M.A. and K.S.S. conceived the project, searched and collected relevant articles from the literature. N.D.P.C. acquired and interpreted the images, and drafted the initial manuscript. All authors reviewed, edited, proof-read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
None
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Concepcion, N.D.P., Laya, B.F., Andronikou, S. et al. Imaging recommendations and algorithms for pediatric tuberculosis: part 1—thoracic tuberculosis. Pediatr Radiol 53, 1773–1781 (2023). https://doi.org/10.1007/s00247-023-05654-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-023-05654-1