Abstract
Background
Permissive hypercapnia is a ventilatory strategy used to prevent lung injury in ventilated extremely low birth weight (ELBW, birth weight ≤1,000 g) infants. However, there is retrospective evidence showing that high CO2 is associated with brain injury.
Objective
The objective of this study was to compare brain white matter development at term-equivalent age in ELBW infants randomized to hypercapnic vs. normocapnic ventilation during the first week of life and in healthy non-ventilated term newborns.
Materials and methods
Twenty-two ELBW infants from a randomized controlled trial were included in this study; 11 received hypercapnic (transcutaneous PCO2 [tcPCO2] 50–60 mmHg) ventilation and 11 normocapnic (tcPCO2 35–45 mmHg) ventilation during the first week of life while still intubated. In addition, ten term healthy newborns served as controls. Magnetic resonance imaging (MRI) with diffusion tensor imaging (DTI) was performed at term-equivalent age for the ELBW infants and at approximately 2 weeks of age for the control infants. White matter injury on conventional MRI was graded in the ELBW and control infants using a scoring system adopted from literature. Tract-based spatial statistics (TBSS) was used to evaluate for differences in DTI measured fractional anisotropy (FA, spatially normalized to a customized template) among the ELBW and term control infants.
Results
Conventional MRI white matter scores were not different (7.3 ± 1.7 vs. 6.9 ± 1.4, P = 0.65) between the hypercapnic and normocapnic ELBW infants. TBSS analysis did not show significant differences (P < 0.05, corrected) between the two ELBW infant groups, although before multiple comparisons correction, hypercapnic infants had many regions with lower FA and no regions with higher FA (P < 0.05, uncorrected) compared to normocapnic infants. When compared to the control infants, normocapnic ELBW infants had a few small regions with significantly lower FA, while hypercapnic ELBW infants had more widespread regions with significantly lower FA (P < 0.05, fully corrected for multiple comparisons).
Conclusions
Normocapnic ventilation vs. permissive hypercapnia may be associated with improved white matter development at term-equivalent age in ELBW infants. This effect, however, was small and was not apparent on conventional MRI. Further research is needed using larger sample sizes to assess if permissive hypercapnic ventilation in ELBW infants is associated with worse white matter development.
Similar content being viewed by others
Introduction
Approximately 30,000 extremely low birth weight (ELBW, birth weight ≤1,000 g) infants are born in the United States each year [1]. Many of them receive mechanical ventilation during the first days or weeks after birth. Neonatologists commonly use permissive hypercapnia, a ventilatory strategy that uses high arterial partial pressure of carbon dioxide (PaCO2) to avoid ventilator-induced lung injury in premature infants [2, 3]. Despite the theoretical benefits to the lung of using lower ventilator tidal volumes and mean airway pressures, randomized controlled trials of permissive hypercapnia failed to show improvement in chronic lung disease rates over infants managed with routine ventilation [4–6].
PaCO2 is a potent regulator of cerebral blood flow [7] and both extremes of PaCO2 are known to adversely affect the premature brain. Hypocapnia has been associated with increased risk of periventricular leukomalacia (PVL) [8–10] while hypercapnia has been associated with developing severe intraventricular hemorrhage (IVH) [11, 12]. The effect of mild hypercapnia on the brain during permissive hypercapnic ventilation in premature infants is less clear. Importantly, cerebral white matter injury frequently accompanies IVH, and is the lesion most associated with poor long-term neurodevelopmental outcomes in preterm infants [13]. The immature development and regulation of the vascular supply to the white matter in ELBW infants suggests a very small margin of safety when cerebral blood flow changes [14, 15]. Although permissive hypercapnia trials using cranial US reported no effects of hypercapnia vs. normocapnia on development of cystic PVL [4, 5], evaluation of subtle white matter abnormality by US is not as sensitive as by MRI [16–19].
Diffusion tensor imaging (DTI) is an MRI technique that is very sensitive to white matter microstructures. DTI uses water diffusion anisotropy to produce contrast for white matter imaging [20]. DTI parameters, such as fractional anisotropy (FA), have shown great sensitivity to white matter injury [21, 22]. Region of interest (ROI) studies in preterm newborns have shown that DTI parameters at term-equivalent age are effective markers for white mater maturation and are predictive of neurodevelopmental outcome [23–26]. Moreover, studies using voxel-wise analysis by tract-based spatial statistics (TBSS) are ROI-independent thus nonsubjective and have also reported great sensitivity in detecting white matter abnormalities in premature infants [27, 28].
In this neuroimaging study, we used conventional MRI and DTI-TBSS to evaluate the white matter development at term-equivalent age in ELBW infants randomized to hypercapnic (transcutaneous PCO2 [tcPCO2] 50–60 mmHg) vs. normocapnic (tcPCO2 35–45 mmHg) ventilation during the first week of life, and compared the TBSS results to healthy term newborns.
Materials and methods
Subjects
ELBW infants with birth weight 401–1,000 g (gestational age <30 weeks) born at the University of Arkansas for Medical Sciences (UAMS) and managed at UAMS and the Arkansas Children’s Hospital (ACH) neonatal intensive care units (NICUs) were recruited from the research population of a clinical trial of permissive hypercapnic vs. normocapnic ventilation. Infants with complex congenital anomalies, central nervous system malformations, chromosomal abnormalities or hydrops fetalis were not enrolled. All of the study infants had birth weight appropriate for gestational age (AGA). Informed consent was obtained from the infants’ parents, and all procedures complied with the UAMS IRB regulations. Twenty-two ELBW infants were included in this study; 11 (four males, seven females) were randomized to hypercapnic ventilation (tcPCO2 50–60 mmHg) and 11 (six males, five females) to normocapnic ventilation (35–45 mmHg) during the first week of life, while still intubated. In addition, ten term healthy newborns with no clinical indications for MRI were recruited from another ongoing trial of neonatal growth at the Arkansas Children’s Nutrition Center and served as controls.
Ventilation protocol for the ELBW infants
After delivery, ELBW infants were brought to a designated resuscitation room in the Labor and Delivery suite for initial newborn resuscitation and stabilization. Attending neonatologists were present at all newborn resuscitations of premature infants. If necessary, infants were intubated, placed on conventional mechanical ventilators (Dräger Babylog 8000 plus ventilator; Dräger Medical, Lübeck, Germany), administered prophylactic surfactant ≤20 min of life and had umbilical catheters placed. Routine intensive care procedures, including repeat surfactant administration, tracheal suctioning, determination of arterial blood gases, intravenous and enteral nutrition schedules and use of sedatives/analgesics were at the discretion of the attending neonatologist.
After informed consent was obtained (usually within 1–3 h after birth), a sealed opaque envelope was opened revealing random assignment to hypercapnic or normocapnic ventilation. Infants were begun on an assist-control volume guarantee mode. For hypercapnic ventilation (tcPCO2 50–60 mmHg and pH ≥7.20), initial tidal volume was set at 5, 4.5 and 4 ml/kg, respectively, for infants with birth weights 401–500, 501–750 and 751–1,000 g. For normocapnic ventilation (tcPCO2 35–45 mmHg and pH ≥7.25), initial tidal volume was set at 5.5, 5 and 4.5 ml/kg, respectively, for infants with birth weights 401–500, 501–750 and 751–1,000 g. The ventilator rate was set at 40–60 breaths/min, inspiratory time at 0.25 s, and positive end-expiratory pressure at 4–5 cm H2O. Weaning protocols from the ventilator, for extubation and for the possible need for reintubation were based on preset study criteria.
MRI examinations
When the ELBW infants were clinically stable and at term-equivalent age, they were transported to the ACH Radiology MRI suite for a no-sedation brain MRI. They were fed in the MRI suite ~30 min before the scan, swaddled in warm sheets and immobilized using a MedVac Infant Immobilizer (CFI Medical Solutions, Fenton, MI). The MRI examinations were performed on a 1.5-T Achieva MRI scanner (Philips Healthcare, Best, the Netherlands) with 60 cm bore size, 33 mT/m gradient amplitude and 100 mT/m/ms maximum slew rate. A pediatric 8-channel sensitivity encoding (SENSE) head coil was used. An MRI-compatible camera was attached to the head coil and the infants were connected to a heart rate and oxygen saturation monitor. Our no-sedation neonatal brain conventional MRI protocol was used, which included sagittal T1-weighted 3-D reconstructed to three planes, axial T2-weighted, axial diffusion-weighted, and axial T2*- weighted or susceptibility-weighted imaging sequences. In addition, a single-shot spin-echo planar imaging sequence with acquisition voxel size 2 × 2 × 3 mm and diffusion-weighting gradients (b = 700 s/mm2) uniformly distributed in 15 directions was used to acquire DTI data.
At postnatal age of approximately 2 weeks, the control newborns underwent the same no-sedation brain MRI examination with the same experimental procedures and DTI sequences as the ex-ELBW infants.
Conventional MRI white matter grading
Conventional MR images for the ELBW infants were transmitted to the hospital picture archiving and communication system (PACS) and were evaluated by two experienced neuroradiologists (C.M.G. and R.H.R.) with 28 and 5 years of experience, respectively, who were blinded to the ventilation group. They independently scored the white matter for each infant, according to the method of Woodward et al. [29]. The scoring consisted of six components: white matter signal intensity on T1 and T2, volume of periventricular white matter, presence of cysts, ventricular dilation, abnormality on DWI, and corpus callosum thickness, with each component scored from 1 to 4, corresponding to normal, mildly, moderately or severely affected, respectively. The overall white matter score was the sum of the six subcategory scores, and the scores from the two neuroradiologists were averaged. In addition, the white matter for each control newborn was also scored using the same mechanism (two control newborns did not have valid axial T2-weighed images and DTI images without diffusion weighting were used instead). The two neuroradiologists also independently evaluated whether there was blood product deposition in the ELBW infants based on conventional MRI, particularly on the T2* weighted gradient echo or susceptibility weighted images, which are very sensitive to blood.
DTI data processing
The FA maps for each infant were calculated from the diffusion tensor images on the scanner. The maps were then exported to a workstation with FMRIB Software Library (FSL; Analysis Group, FMRIB, Oxford, UK). Voxel-wise analysis of the FA data was carried out using TBSS [28]. Each FA dataset was aligned to every other one to identify the most representative one (which had the minimal amount of total warping) that consequently served as the target, and nonlinear transforms were performed to register each FA dataset to this target. Spatial normalization to a standard template (e.g., MN152) was not performed because brain structures in ELBW infants differ greatly from adults. Instead, the target FA dataset served as a customized template. After that, all FA images were merged and fed into the FA skeletonisation program to create a mean FA skeleton in which a threshold of FA ≥0.15 was chosen. Mean FA value of brain white matter for each infant was calculated on the FA skeleton. Randomization with Threshold-Free Cluster Enhancement (TFCE) was used to perform voxel-wise comparison of FA values between the hypercapnic and normocapnic ELBW infants and the control newborns.
Statistics
For comparison of demographic parameters and white matter scores by conventional MRI for the hypercapnic and normocapnic infants and control newborns, values are reported as mean ± standard deviation; a Wilcoxon rank sum test was used to determine if there were group differences. P < 0.05 was regarded as significant. For the DTI-TBSS analysis, the TFCE P < 0.05 before and after multiple comparisons correction was used for the voxel-wise comparisons to detect regions with significant difference in FA values between groups.
Results
The demographic information and conventional MRI findings for all infants are listed in Table 1. The gestational and postmenstrual ages at MRI were similar between the hypercapnic and normocapnic ELBW infants (25.5 ± 0.9 vs. 25.0 ± 1.4 weeks, P = 0.37; 41.7 ± 3.2 vs. 40.1 ± 1.5 weeks, P = 0.26, respectively). For control infants, the gestational age was 39.0 ± 0.9 weeks, and the postmenstrual age at MRI was similar to that of the ELBW infants (41.2 ± 1.0 vs. 40.9 ± 2.6 weeks, P = 0.24). The hypercapnic and normocapnic ELBW infants had comparable median (25th, 75th percentile) 1-min (2 [1, 4] vs. 1 [1, 2], P = 0.20) and 5-min Apgar (6 [4, 7] vs. 6 [4, 6], P = 0.76) scores.
None of the ELBW infants had acute bleeding in the brain, however 9 hypercapnic and 6 normocapnic infants (P = 0.17, chi-square test) showed blood product deposition on MRI at term-equivalent age, indicating previous cerebral hemorrhage. The average white matter score by conventional MRI was similar for the hypercapnic and normocapnic infants (7.3 ± 1.7 vs. 6.9 ± 1.4, P = 0.65), and was higher (indicating apparent white matter injury) in ELBW infants compared to newborn controls (7.1 ± 1.5 vs. 6.0 ± 0.0, P = 0.005). The observed white matter abnormalities in ELBW infants included abnormal signal intensity on T1- and T2-weighted images (in four hypercapnic and four normocapnic ELBW infants), decreased volume of periventricular white matter (two hypercapnic and one normocapnic ELBW infants), presence of cysts (one hypercapnic and one normocapnic ELBW infants), mild ventricular dilatation (three hypercapnic and one normocapnic ELBW infants), and thinning of the corpus callosum (five hypercapnic and two normocapnic ELBW infants). The correlation between white matter score and mean FA value for whole brain was negative but not significant (r = −0.14, P = 0.53, Spearman correlation test) in ELBW infants.
Voxel-wise TBSS analysis revealed that the hypercapnic infants had lower FA values (P < 0.05, before adjusting for multiple comparisons) in multiple white matter regions compared to the normocapnic infants, as illustrated in Fig. 1. These differences, however, did not survive multiple comparisons correction. On the other hand, there were no white matter regions that had higher FA values (P < 0.05, before adjusting for multiple comparisons) in the hypercapnic infants.
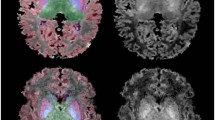
TBSS analysis between the term control infants and the ELBW infants (Fig. 2) revealed that the hypercapnic infants had multiple white matter regions with significantly lower FA values (P < 0.05, fully corrected for multiple comparisons across space) compared to control infants, including most of the genu, body and splenium of the corpus callosum; normocapnic ELBW infants, on the other hand, had far fewer regions with significantly lower FA values (P < 0.05, fully corrected for multiple comparisons across space) compared to controls, mainly in the splenium of corpus callosum.
TBSS analysis for hypercapnic ELBW infants (a) compared to term control infants shows widespread white matter regions with significantly decreased FA (orange/yellow, P < 0.05, fully corrected for multiple comparisons across space); TBSS analysis for normocapnic ELBW infants (b) compared to term control infants shows fewer regions with significantly decreased FA (orange/yellow, P < 0.05, fully corrected for multiple comparisons across space)
Discussion
While conventional MRI is adequate to evaluate overt white matter injury in premature infants, DTI has superior sensitivity to subtle white matter abnormalities. DTI studies have reported abnormal white matter development in premature children at different ages [27, 30], and DTI parameters correlated with neurodevelopmental outcome [31, 32]. Studies using DTI-TBSS combined with ROI analysis have found decreased FA values in premature infants at term-equivalent age compared to term control infants [27]; our findings are similar. In both the hypercapnic and normocapnic ELBW infants, decreased FA values were found in the corpus callosum, the largest and one of the most rapidly developing white matter structures in the brain during the late prenatal period. Since myelination of the corpus callosum mainly occurs at 3–6 months corrected age [33], the decrease in FA values in the corpus callosum in the term-equivalent ELBW infants may be associated with incomplete development of axonal bundles or injury to pre-myelinating oligodendrocytes.
Hypercapnia increases cerebral blood flow by promoting vascular smooth muscle relaxation. Further, the CO2 reactivity of the cerebral vasculature in premature infants is adult-like [34] and fluctuations in PCO2, which are common in ventilated ELBW infants undergoing intensive care, may lead to changes in cerebral blood flow that are potentially detrimental to the vulnerable brain in ELBW infants. These may be especially detrimental to the germinal matrix, which has fragile thin-walled vessels and is the usual origin of IVH in preterm newborns [35]. The germinal matrix destruction caused by IVH may induce deficits of white matter, since the germinal matrix provides glial precursor cells that become oligodendroglia and astrocytes, which are important for the development of white matter. Studies have shown that extreme hypercapnia is a risk factor for severe IVH in extremely premature infants [11, 12]. Additionally, there have been three randomized controlled trials of permissive hypercapnia vs. normocapnic ventilation in premature infants: The first pilot trial reported a nonsignificant increase in IVH in hypercapnic (45–55 vs. 35–45 mmHg) infants [4] and conversely, the second minimal ventilation trial observed a nonsignificant increase in IVH in infants managed with lower targeted PCO2 (<48 vs. >52 mmHg) [5]. A third trial reported worse mental development in ELBW infants managed with higher targeted PCO2 (55–65 vs. 35–45 mmHg) [6]. Nevertheless, whether permissive hypercapnia has subtle effects on white matter development in ELBW infants was unknown and previously not studied. In our study using conventional MRI, we found no difference in white matter injury between ELBW infants randomized to hypercapnic vs. normocapnic ventilation. However, with advanced neuroimaging techniques such as DTI, which is more sensitive to subtle white matter injury, and TBSS, which provides whole-brain nonsubjective voxel-wise analysis, we found hypercapnic ELBW infants had more extensive white matter developmental abnormalities (e.g., in both genu and splenium of corpus callosum) at term-equivalent age compared to term healthy newborns, while normocapnic ELBW infants had less abnormalities (e.g., only in the splenium of corpus callosum). The different extents of abnormality in genu and splenium may be related to density of white matter (splenium usually has slightly higher FA values than genu in infants) or timing of development (splenium usually myelinates earlier than genu), and may reflect secondary injury by permissive hypercapnia. Previous studies have revealed the association between hypocapnia and apparent white matter injury such as PVL [8–10] in premature infants, presumably as a consequence of hypoxic ischemic injury due to severe vasoconstriction. On the other hand, vasodilation and excessively increased cerebral blood flow by permissive hypercapnia may also induce risk of cerebral hemorrhage and white matter injury, as our results indicated a trend for a higher rate of blood product deposition (82% vs. 55%) and that normocapnia, instead of hypercapnia, is associated with minimal white matter developmental abnormalities in ELBW infants at term-equivalent age compared to term control newborns.
The main limitation of this study is the small sample size. We enrolled ventilation-wise well-characterized ELBW infants from a randomized controlled trial, and prospectively evaluated their brains by MRI at term-equivalent age. Some of the infants in the larger study were not physiologically stable at term-equivalent age, and MRIs could not be completed at the specified time. In addition, there were five more ELBW infants (three hypercapnic and two normocapnic) who completed the MRI but were excluded from this study because of moderate to severe ventricular dilatation (as determined by conventional MRI scores), which largely distorted brain white matter anatomy and caused artifact to the TBSS analysis. Mild ventricular dilatation and other brain abnormalities such as PVL and IVH did not affect the normalization in TBSS analysis, as determined by slice-by-slice review of imaging registration. The no-sedation MRI protocol also restricted our scan time and the number of pulse sequences that could be used in the ELBW and control infants. For example, our DTI sequence for all infants was short, and controls did not have separate DWI and gradient echo or susceptibility weighted images. Nevertheless, DTI-TBSS did not require high numbers of diffusion directions and the quality of our FA maps and the following registration were satisfactory. For the controls, diffusion- weighted images were obtained from DTI for the conventional MRI scoring of white matter, and blood product deposition determination was not performed. With our sample size and statistical power, we only observed uncorrected white matter FA differences between the hypercapnic and normocapnic ELBW infants in the DTI-TBSS analysis. However, the differences in white matter FA were prominent when compared to healthy control infants respectively (with multiple comparisons correction). Finally, lack of neurodevelopmental outcome data is also a limitation of this study. Additional studies with larger sample sizes will be necessary to assess the effect of permissive hypercapnia vs. normocapnic ventilation on white matter development and neurodevelopmental outcome in ELBW infants.
Conclusion
In this study, we evaluated the effects of permissive hypercapnia on cerebral white matter in ELBW infants by conventional MRI and DTI-TBSS analysis. Our results indicated that there were subtle differences in white matter development that were not apparent on conventional MRI, and that normocapnia instead of permissive hypercapnia may be beneficial to white matter development at term-equivalent age in ventilated ELBW infants.
References
Martin JA, Hamilton BE, Ventura SJ et al (2012) Births: final data for 2010. Natl Vital Stat Rep 61:1–71
Thome UH, Ambalavanan N (2009) Permissive hypercapnia to decrease lung injury in ventilated preterm neonates. Semin Fetal Neonatal Med 14:21–27
Thome UH, Carlo WA (2002) Permissive hypercapnia. Semin Neonatol 7:409–419
Mariani G, Cifuentes J, Carlo WA (1999) Randomized trial of permissive hypercapnia in preterm infants. Pediatrics 104:1082–1088
Carlo WA, Stark AR, Wright LL et al (2002) Minimal ventilation to prevent bronchopulmonary dysplasia in extremely-low-birth-weight infants. J Pediatr 141:370–374
Thome UH, Carroll W, Wu TJ et al (2006) Outcome of extremely preterm infants randomized at birth to different PaCO2 targets during the first seven days of life. Biol Neonate 90:218–225
Brian JE (1998) Carbon dioxide and the cerebral circulation. Anesthesiology 88:1365–1386
Fujimoto S, Togari H, Yamaguchi N et al (1994) Hypocapnia and cystic periventricular leukomalacia in premature-infants. Arch Dis Child 71:F107–F110
Okumura A, Hayakawa F, Kato T et al (2001) Hypocarbia in preterm infants with periventricular leukomalacia: the relation between hypocarbia and mechanical ventilation. Pediatrics 107:469–475
Wiswell TE, Graziani LJ, Kornhauser MS et al (1996) Effects of hypocarbia on the development of cystic periventricular leukomalacia in premature infants treated with high-frequency jet ventilation. Pediatrics 98:918–924
Kaiser JR, Gauss CH, Pont MM et al (2006) Hypercapnia during the first 3 days of life is associated with severe intraventricular hemorrhage in very low birth weight infants. J Perinatol 26:279–285
Fabres J, Carlo WA, Phillips V et al (2007) Both extremes of arterial carbon dioxide pressure and the magnitude of fluctuations in arterial carbon dioxide pressure are associated with severe intraventricular hemorrhage in preterm infants. Pediatrics 119:299–305
Perlman JM (1998) White matter injury in the preterm infant: an important determination of abnormal neurodevelopment outcome. Early Hum Dev 53:99–120
Volpe JJ (2001) Neurobiology of periventricular leukomalacia in the premature infant. Pediatr Res 50:553–562
Volpe JJ (2001) Neurology of the newborn, 4th edn. W.B. Saunders Co., Philadelphia
Childs AM, Cornette L, Ramenghi LA et al (2001) Magnetic resonance and cranial ultrasound characteristics of periventricular white matter abnormalities in newborn infants. Clin Radiol 56:647–655
Debillon T, N’Guyen S, Muet A et al (2003) Limitations of ultrasonography for diagnosing white matter damage in preterm infants. Arch Dis Child Fetal Neonatal Ed 88:F275–F279
Inder TE, Anderson NJ, Spencer C et al (2003) White matter injury in the premature infant: a comparison between serial cranial sonographic and MR findings at term. AJNR Am J Neuroradiol 24:805–809
Miller SP, Cozzio CC, Goldstein RB et al (2003) Comparing the diagnosis of white matter injury in premature newborns with serial MR imaging and transfontanel ultrasonography findings. AJNR Am J Neuroradiol 24:1661–1669
Basser PJ, Mattiello J, LeBihan D (1994) MR diffusion tensor spectroscopy and imaging. Biophys J 66:259–267
Inder T, Huppi PS, Zientara GP et al (1999) Early detection of periventricular leukomalacia by diffusion-weighted magnetic resonance imaging techniques. J Pediatr 134:631–634
Nakayama N, Okumura A, Shinoda J et al (2006) Evidence for white matter disruption in traumatic brain injury without macroscopic lesions. J Neurol Neurosurg Psychiatry 77:850–855
Arzoumanian Y, Mirmiran M, Barnes PD et al (2003) Diffusion tensor brain imaging findings at term-equivalent age may predict neurologic abnormalities in low birth weight preterm infants. AJNR Am J Neuroradiol 24:1646–1653
Dudink J, Lequin M, van Pul C et al (2007) Fractional anisotropy in white matter tracts of very-low-birth-weight infants. Pediatr Radiol 37:1216–1223
Huppi PS, Murphy B, Maier SE et al (2001) Microstructural brain development after perinatal cerebral white matter injury assessed by diffusion tensor magnetic resonance imaging. Pediatrics 107:455–460
Partridge SC, Mukherjee P, Henry RG et al (2004) Diffusion tensor imaging: serial quantitation of white matter tract maturity in premature newborns. Neuroimage 22:1302–1314
Anjari M, Srinivasan L, Allsop JM et al (2007) Diffusion tensor imaging with tract-based spatial statistics reveals local white matter abnormalities in preterm infants. Neuroimage 35:1021–1027
Smith SM, Jenkinson M, Johansen-Berg H et al (2006) Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 31:1487–1505
Woodward LJ, Anderson PJ, Austin NC et al (2006) Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N Engl J Med 355:685–694
Skranes J, Vangberg TR, Kulseng S et al (2007) Clinical findings and white matter abnormalities seen on diffusion tensor imaging in adolescents with very low birth weight. Brain 130:654–666
Rose J, Butler EE, Lamont LE et al (2009) Neonatal brain structure on MRI and diffusion tensor imaging, sex, and neurodevelopment in very-low-birthweight preterm children. Dev Med Child Neurol 51:526–535
van Kooij BJM, de Vries LS, Ball G et al (2012) Neonatal tract-based spatial statistics findings and outcome in preterm infants. AJNR Am J Neuroradiol 33:188–194
Deoni SCL, Mercure E, Blasi A et al (2011) Mapping infant brain myelination with magnetic resonance imaging. J Neurosci 31:784–791
Kaiser JR, Gauss CH, Williams DK (2004) Surfactant administration acutely affects cerebral and systemic hemodynamics and gas exchange in very-low-birth-weight infants. J Pediatr 144:809–814
Hambleton G, Wigglesworth JS (1976) Origin of intraventricular haemorrhage in the preterm infant. Arch Dis Child 51:651–659
Acknowledgments
Ou was supported by the Children’s University Medical Group (CUMG) at UAMS and the Thrasher Research Fund. Mulkey was supported by the Center for Translational Neuroscience award from the National Institutes of Health (P20 GM103425). Kaiser was supported by the National Institutes of Health (1K23NS43185, RR20146 and 1R01NS060674) and the UAMS Translational Research Institute (1UL1RR029884). The technical assistance of Natalie C. Sikes, Melanie J. Mason and Nicole A. Johnson and the support of the UAMS and ACH neonatologists, NICU nurses, respiratory therapists, and MRI technologists and nurses are gratefully appreciated. Ou was additionally mentored by Thomas Badger, PhD, and Michael Borrelli, PhD, for this project.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ou, X., Glasier, C.M., Ramakrishnaiah, R.H. et al. Diffusion tensor imaging in extremely low birth weight infants managed with hypercapnic vs. normocapnic ventilation. Pediatr Radiol 44, 980–986 (2014). https://doi.org/10.1007/s00247-014-2946-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-014-2946-8