Abstract
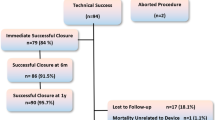
Transcatheter closure of perimembranous ventricular septal defect (PmVSD) is an established procedure. However, the occurrence of complete heart block limits its scope. The newer KONAR-MF™ occluder has specific design characteristics that may improve the safety of PmVSD closure. The objective of the study was to describe the efficacy and mid-term follow-up of transcatheter closure of PmVSD using KONAR-MF™. The study was conducted prospectively in 3 Indian centers (January 2018–December 2022). PmVSD closure was done by both antegrade and retrograde methods, and patients were followed up at 1, 3, 6, 12 months, and annually after that. 121 out of 123 patients were included with the following characteristics: median age 4.4 (0.18–40) years; weight 15 (2.1–88) kg; mean Qp/Qs ratio 1.87 ± 0.52 and pulmonary artery mean pressure: 22 ± 6.9 mmHg. The procedure was successful in all but 3; the device was removed due to significant residual shunt (n = 2) and new development of aortic regurgitation (AR) (≥ mild) in 1. The median defect size was 5.2 (2.5–12) mm. Device sizes from 6/4 to 14/12 were deployed (median fluoroscopy time 13.3 min; range 3.6–47.8). Shunt occlusion rates were 90%-Immediate, 95%-pre-discharge, and 97%-1 month, with no instances of complete heart block after the procedure and during follow-up. Six had new onset AR (mild: 2, trivial 4), and one had increased tricuspid regurgitation. All patients were well during follow-up (median: 20 months; range: 6–46). The new KONAR-MF™ occluder appears to be a promising and safe alternative for the closure of the PmVSD; further long-term follow is merited.




Similar content being viewed by others
Data Availability
No datasets were generated or analysed during the current study.
References
Cohen MS, Lopez L (2016) Ventricular septal defect. In: Allen HD, Shaddy RE, Penny DJ, Feltes TF, Cetta F (eds) Moss and adams’ heart disease in infants, children, and adolescents, 9th edn. Williams and Wilkins, Baltimore, pp 783–801
Anderson R, Lenox C, Zuberbuhler J (1983) Mechanisms of closure of perimembranous ventricular septal defect. Am J Cardiol 52(3):341–345
Krovetz L (1998) Spontaneous closure of ventricular septal defect. Am J Cardiol 81(1):100–101
Keith JD, Rose V, Collins G, Kidd BS (1971) Ventricular septal defect. Incidence, morbidity, and mortality in various age groups. Br Heart J 33:81–7
Kirklin J, Kouchoukos N (2013) Kirklin/Barratt-Boyes cardiac surgery. Elsevier/Saunders, Philadelphia, p 16
Corone P, Doyon F, Gaudeau S, Guérin F, Vernant P, Ducam H et al (1977) Natural history of the ventricular septal defect. A study involving 790 cases. Circulation 55(6):908–15
Van Hare GF, Soffer LJ, Sivakoff MC, Liebman J (1987) Twenty-five-year experience with ventricular septal defect in infants and children. Am Heart J 114(3):606–614
Yeager SB, Freed MD, Keane JF, Norwood WI, Castaneda AR (1984) Primary surgical closure of ventricular septal defect in the first year of life: results in 128 infants. J Am Coll Cardiol 3:1269–1276
Fu YC, Bass J, Amin Z, Radtke W, Cheatham JP, Hellenbrand WE, Balzer D, Cao QL, Hijazi ZM (2006) Transcatheter closure of perimembranous ventricular septal defects using the new Amplatzer membranous VSD occluder: results of the US phase I trial. J Am Coll Cardiol 47(2):319–25. https://doi.org/10.1016/j.jacc.2005.09.028
Predescu D, Chaturvedi RR, Friedberg MK, Benson LN, Ozawa A, Lee KJ (2008) Complete heart block associated with device closure of perimembranous ventricular septal defects. J Thorac Cardiovasc Surg 136(5):1223–1228. https://doi.org/10.1016/j.jtcvs.2008.02.037
Schubert S, Kelm M, Koneti NR, Berger F (2019) First European experience of percutaneous closure of ventricular septal defects using a new CE-marked VSD occluder. EuroIntervention 15(3):e242–e243. https://doi.org/10.4244/EIJ-D-18-00867
Haddad RN, Daou LS, Saliba ZS (2020) Percutaneous closure of restrictive-type perimembranous ventricular septal defect using the new KONAR multifunctional occluder: midterm outcomes of the first middle-eastern experience. Catheter Cardiovasc Interv 96(3):E295–E302. https://doi.org/10.1002/ccd.28678
Tanidir IC, Baspinar O, Saygi M, Kervancioglu M, Guzeltas A, Odemis E (2020) Use of Lifetech™ Konar-MF, a device for both perimembranous and muscular ventricular septal defects: a multicentre study. Int J Cardiol 1(310):43–50. https://doi.org/10.1016/j.ijcard.2020.02.056
Sadiq M, Qureshi AU, Younas M, Arshad S, Hyder SN (2022) Percutaneous closure of ventricular septal defect using LifeTech™ Konar-MF VSD Occluder: initial and short-term multi-institutional results. Cardiol Young 32(5):755–761. https://doi.org/10.1017/S1047951121002985
Odemis E, Kizilkaya MH (2023) Early and mid-term outcomes of transcatheter closure of perimembranous ventricular septal defects using the Lifetech™ Konar-MF occluder device (MFO). Cardiol Young 33(10):2021–2026. https://doi.org/10.1017/S1047951122003547
Koneti NR, Sreeram N, Penumatsa RR, Arramraj SK, Karunakar V, Trieschmann U (2012) Transcatheter retrograde closure of perimembranous ventricular septal defects in children with the Amplatzer duct occluder II device. J Am Coll Cardiol 60(23):2421–2422. https://doi.org/10.1016/j.jacc.2012.08.1004
Koneti NR, Penumatsa RR, Kanchi V, Arramraj SK, Bhupathiraju S (2011) Retrograde transcatheter closure of ventricular septal defects in children using the Amplatzer Duct Occluder II. Catheter Cardiovasc Interv 77(2):252–259. https://doi.org/10.1002/ccd.22675
Fischer G, Apostolopoulou SC, Rammos S, Schneider MB, Bjørnstad PG, Kramer HH (2007) The Amplatzer Membranous VSD Occluder and the vulnerability of the atrioventricular conduction system. Cardiol Young 17(5):499–504. https://doi.org/10.1017/S1047951107000984
Carminati M, Butera G, Chessa M, De Giovanni J, Fisher G, Gewillig M, Peuster M, Piechaud JF, Santoro G, Sievert H, Spadoni I, Walsh K (2007) Investigators of the European VSD registry. Transcatheter closure of congenital ventricular septal defects: results of the European registry. Eur Heart J 28(19):2361–2368. https://doi.org/10.1093/eurheartj/ehm314
Butera G, Carminati M, Chessa M, Piazza L, Micheletti A, Negura DG, Abella R, Giamberti A, Frigiola A (2007) Transcatheter closure of perimembranous ventricular septal defects: early and long-term results. J Am Coll Cardiol 50(12):1189–1195. https://doi.org/10.1016/j.jacc.2007.03.068
Latham RA, Anderson RH (1972) Anatomical variations in atrioventricular conduction system with reference to ventricular septal defects. Br Heart J 34(2):185–190
Bass JL, Kalra GS, Arora R, Masura J, Gavora P, Thanopoulos BD, Torres W, Sievert H, Carminati M, Fischer G, Ewert P (2003) Initial human experience with the Amplatzer perimembranous ventricular septal occluder device. Catheter Cardiovasc Interv 58(2):238–245. https://doi.org/10.1002/ccd.10406
Swartbol P, Truedsson L, Pärsson H, Norgren L (1997) Tumor necrosis factor-alpha and interleukin-6 release from white blood cells induced by different graft materials in vitro are affected by pentoxifylline and iloprost. J Biomed Mater Res 36(3):400–406
Voûte MT, Bastos Gonçalves FM, van de Luijtgaarden KM, Klein Nulent CG, Hoeks SE, Stolker RJ, Verhagen HJ (2012) Stent graft composition plays a material role in the postimplantation syndrome. J Vasc Surg 56(6):1503–1509. https://doi.org/10.1016/j.jvs.2012.06.072
Nageswara Rao Koneti (2023) Multi-functional occluder, Indian patent no: 420711; Intellectual property of India
Kaur D, Rajan S, Shah M, Koneti NR, Calambur N (2022) Mapping the conduction system in patients undergoing transcatheter device closure of perimembranous ventricular septal defect: a proof-of-concept study. Pediatr Cardiol 43(3):674–684. https://doi.org/10.1007/s00246-021-02773-0
Ece İ, Bağrul D, Kavurt AV, Terin H, Torun G, Koca S, Gül AEK (2024) Transcatheter ventricular septal defect closure with Lifetech™ Konar-MF Occluder in infants under 10 kg with only using venous access. Pediatr Cardiol 45(1):175–183. https://doi.org/10.1007/s00246-023-03350-3
Kuswiyanto RB, Gunawijaya E, Djer MM, Noormanto RMA, Murni IK, Sukardi R, Utamayasa A, Ardiansyah R, Nova R, Liliyanti S, Rahayuningsih SE, Anggriawan SL, Rahayuningsih TY, Koentartiwi D, Soewarniaty R, Yantie VK, Nugroho S, Hidayat T, Ontoseno T, Tobing TC, Ali M, Bashari MH, Yosy DS, Arafuri N, Hilmanto D, Yanuarso PB, Advani N, Sastroasmoro S, Putra ST (2022) Transcatheter closure of perimembranous ventricular septal defect using the Lifetech Konar-multi functional occluder: early to midterm results of the indonesian multicenter study. Glob Heart 17(1):15. https://doi.org/10.5334/gh.1106
Author information
Authors and Affiliations
Contributions
K.N, S.Z, K.K wrote manuscript R.S: study design and revision S.B, B.D: statistics and prepared figures.
Corresponding author
Ethics declarations
Conflict of interest
Nageswara Rao Koneti is the inventor of the KONAR—MF device and receives a royalty from Lifetech Scientific, Schengen. The multi-functional occluder has patents from India 420711, Euro-Asia 036309, and Korea 10-389237.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Koneti, N.R., Azad, S., Bakhru, S. et al. Transcatheter Closure of Perimembranous Ventricular Septal Defect Using KONAR-MF™: A Multicenter Experience. Pediatr Cardiol (2024). https://doi.org/10.1007/s00246-024-03505-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00246-024-03505-w