Abstract
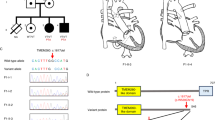
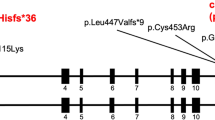
Patent ductus arteriosus (PDA) is a common congenital heart disease that develops soon after birth when the arterial duct does not remodel. Mutations in TFAP2B, which encodes a neural crest-derived transcription factor, can cause Char syndrome, characterized by PDA, facial dysmorphism, and skeletal abnormalities of the hand. The TFAP2B mutations result in a great amount of phenotypic variability, and a novel TFAP2B mutation has been found in patients with nonsyndromic PDA. Therefore, this study investigated whether TFAP2B mutations can cause familial nonsyndromic PDA. Clinical data and peripheral blood specimens were collected from two kindreds (A and B) and from a cohort of 100 unrelated subjects with PDA. Kindred A spanned three generations, in which 5 of the 16 individuals had PDA, and kindred B spanned three generations, in which 2 of the 13 individuals had PDA. The study enrolled 100 unrelated healthy individuals as control subjects. Polymerase chain reaction (PCR) was used to amplify seven exons and flanking introns of the TFAP2B gene. A few exons of the TFAP2B gene were amplified using reverse transcription polymerase chain reaction (RT-PCR), and direct forward and reverse sequencing of the PCR products was performed. The acquired sequences were aligned with those in GenBank by using a basic local alignment search tool (BLAST). The following two types of mutations were identified in TFAP2B: c.601+5G>A and c.435_438delCCGG. The mutation c.601+5G>A was detected in the affected members of kindred A. Nested PCR showed a splice junction in intron 3 and a 61-bp deletion in exon 3. The mutation c.435_438delCCGG, found in the affected members of kindred B, was caused by a four-base deletion in exon 2, which in turn caused a frame shift that resulted in the formation of a premature stop codon, p.Arg145Argfsx45. None of these mutations was detected in the unaffected members of the kindred or in the control group. Furthermore, two novel single-nucleotide polymorphisms (SNPs), c.1-34G>A and c.539+62G>C, were detected in the introns. The variant c.1-34G>A was identified 34 bp upstream of the transcription initiation site in the TFAP2B gene. Significant differences in the prevalence of the alleles G and A were observed in the control subjects and PDA patients (Z = −2.513, P = 0.012). The study identified that another variant was c.539+62G>C but that the frequency of this variant was similar between the control subjects and the PDA patients (Z = −0.332, P = 0.74). The TFAP2B mutations may be associated with isolated nonsyndromic, hereditary PDA in Chinese families. The authors propose that a TFAP2B mutation should be considered a risk factor for isolated PDA. However, the detailed genetic mechanism underlying nonsyndromic the PDA-causing TFAP2B mutation is yet to be elucidated.





Similar content being viewed by others
References
Bökenkamp R, Deruiter MC, van Munsteren C, Bökenkamp R, van Munsteren C et al (2010) Insights into the patohgenesis and genetic background of patency of ductus arteriosus. Neonatology 98:6–17
Dagle JM, Lepp NT, Cooper ME et al (2009) Determination of genetic predisposition to patent ductus arteriosus in preterm infants. Pediatrics 123:1116–1123
Forsey JT, Elmasry OA, Martin RP (2009) Patent arterial duct. Orphanet J Rare Dis 4:17
Hamrick SE, Hansmann G (2010) Patent ductus arteriosus of the preterm infant. Pediatrics 125:1020–1030
Hermes-DeSntis ER, Clyman RI (2006) Patent ductus arteriosus: pathophysiology and management. J Perinatol Suppl 1:S13–18; discussion S22–23
Hilger-Eversheim K, Moser M, Schorle H et al (1997) Regulatory roles of AP-2 transcription factors in vertebrate development, apoptosis, and cell-cycle control. Gene 260:1–12
Iver KN, Sutcliffe D, Richardson J et al (2008) Transcriptional regulation during development of the ductus arteriosus. Circ Res 103:388–395
Khetyar M, Syrris P, Tinworth L et al (2008) Novel TFAP2B mutation in nonsyndromic patent ductus arteriosus. Genet Test 12:457–459
Leonhardt A, Glaser A, Wegmann M et al (2003) Expression of prostanoid receptors in human ductus arteriosus. Br J Pharmacol 138:655–659
Mani A, Radhakrishnan J, Farhi A et al (2005) Syndromic patent ductus arteriosus: evidence for haploinsufficient TFAP2B mutations and identification of a linked sleep disorder. Proc Natl Acad Sci USA 102:2975–2979
Moser M, Pscherer A, Roth C et al (1997) Enhanced apoptotic cell death of renal epithelial cells in mice lacking transcription factor AP-2beta. Genes Dev 11:1938–1948
Moser M, Ruschoff J, Buettner R et al (1997) Comparative analysis of AP-2 alpha and AP-2 beta gene expression during murine embryogenesis. Dev Dyn 208:115–124
Moser M, Dahmen S, Kluge R et al (2003) Terminal renal failure in mice lacking transcription factor AP-2 beta. Lab Invest 83:571–578
Satoda M, Pierpont ME, Diaz GA et al (1999) Char syndrome, an inherited disorder with patent ductus arteriosus, maps to chromosome 6p12–p21. Circulation 99:3036–3042
Satoda M, Zhao F, Diaz GA et al (2000) Mutations in TFAP2B cause Char syndrome, a familial form of patent ductus arteriosus. Nat Genet 25:42–46
Schneider DJ, Moore JW (2006) Patent ductus arteriosus. Circulation 114:1873–1882
Tsukada S, Tanaka Y, Maegawa H et al (2006) Intronic polymorphisms within TFAP2B regulate transcriptional activity and affect adipocytokine gene expression in differentiated adipocytes. Mol Endocrinol 20:1104–1111
Waleh N, Hodnick R, Jhaveri N et al (2010) Patterns of gene expression in the ductus arteriosus are related to environmental and genetic risk factors for persistent ducts patency. Pediatr Res 68:292–297
Zhao F, Weismann CG, Satoda M et al (2001) Novel TFAP2B mutations that cause Char syndrome provide a genotype–phenotype correlation. Am J Hum Genet 69:695–703
Acknowledgments
We thank all the patients and their families who participated in this study. We thank all the staff of the Heart Center for their assistance in case collection. This work was supported by the Shanghai Municipal Education Commission Major Project (no. 11ZZ14) and the Shanghai University Innovation Team.
Author information
Authors and Affiliations
Corresponding author
Additional information
Yi-Wei Chen, Wu Zhao contributed equally to this work.
Rights and permissions
About this article
Cite this article
Chen, YW., Zhao, W., Zhang, ZF. et al. Familial Nonsyndromic Patent Ductus Arteriosus Caused by Mutations in TFAP2B . Pediatr Cardiol 32, 958–965 (2011). https://doi.org/10.1007/s00246-011-0024-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-011-0024-7