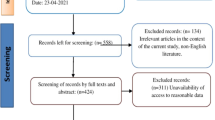
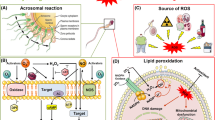
Abstract
The crude extract of Trachyspermum ammi seeds (Ta.Cr) was studied for its antiurolithic activity using the in vivo and in vitro experiments. In the in vivo experiments, Ta.Cr treatment showed a diuretic activity at the dose of 30 and 100 mg/kg and exhibited curative effect in male hyperoxaluric Wistar rats, which received 0.75% ethylene glycol (EG) in drinking water given for 3 weeks, with 1% ammonium chloride (AC) for initial three days. In the in vitro experiments, Ta.Cr delayed the slopes of nucleation and inhibited the calcium oxalate (CaOx) crystal aggregation in a concentration-dependent manner like that of potassium citrate. Ta.Cr also inhibited DPPH free radicals like standard antioxidant drug butylated hydroxytoluene (BHT), and significantly reduced cell toxicity and LDH release in Madin–Darby canine kidney (MDCK) cells, exposed to oxalate (0.5 mM) and COM (66 µg/cm2) crystals. In isolated rabbit urinary bladder strips, Ta.Cr relaxed high K+ (80 mM) and CCh (1 µM)-induced contractions, showing antispasmodic activity. The findings of this study suggest that the antiurolithic activity of crude extract of Trachyspermum ammi seeds may be mediated by a number of mechanisms, including a diuretic, an inhibitor of CaOx crystal aggregation, an antioxidant, renal epithelial cell protection, and an antispasmodic, thus, showing the therapeutic potential in urolithiasis, for which there is no viable non-invasive option in modern medicine.












Similar content being viewed by others
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
References
Lemann J Jr (1993) Composition of the diet and calcium kidney stones. N Engl J Med 328(12):880–882. https://doi.org/10.1056/NEJM199303253281212
Evan AP (2010) Physiopathology and etiology of stone formation in the kidney and the urinary tract. Pediatr Nephrol 25(5):831–841. https://doi.org/10.1007/s00467-009-1116-y
Khan SR (2013) Reactive oxygen species as the molecular modulators of calcium oxalate kidney stone formation: evidence from clinical and experimental investigations. J Urol 189(3):803–811
Khan SR (2012) Is oxidative stress, a link between nephrolithiasis and obesity, hypertension, diabetes, chronic kidney disease, metabolic syndrome? Urol Res 40(2):95–112. https://doi.org/10.1007/s00240-011-0448-9
Tsujihata M, Momohara C, Yoshioka I, Tsujimura A, Nonomura N, Okuyama A (2008) Atorvastatin inhibits renal crystal retention in a rat stone forming model. J Urol 180(5):2212–2217. https://doi.org/10.1016/j.juro.2008.07.024
Gaspar S, Niculite C, Cucu D, Marcu I (2010) Effect of calcium oxalate on renal cells as revealed by real-time measurement of extracellular oxidative burst. Biosens Bioelectron 25(7):1729–1734. https://doi.org/10.1016/j.bios.2009.12.013
Khan SR (2005) Hyperoxaluria-induced oxidative stress and antioxidants for renal protection. Urol Res 33(5):349–357. https://doi.org/10.1007/s00240-005-0492-4
Hollenberg D, Zakus D, Cook T, Xu XW (2008) Re-positioning the role of traditional, complementary and alternative medicine as essential health knowledge in global health: do they still have a role to play? World Health Popul 10(4):62–75
Gilani AH, Rahman AU (2005) Trends in ethnopharmocology. J Ethnopharmacol 100(1–2):43–49. https://doi.org/10.1016/j.jep.2005.06.001
Usmanghani K, Saeed A, Alam MT (1997) Indusyunic medicine. University of Karachi Press, Karachi
Duke JA (2002) Handbook of medicinal herbs. CRC Press
Duke JA (1992) Handbook of phytochemical constituents of GRAS herbs and other economic plants. CRC Press Inc., Boca Raton, pp 292–293
Srivastava KC (1988) Extract of a spice—omum (Trachyspermum ammi)-shows antiaggregatory effects and alters arachidonic acid metabolism in human platelets. Prostaglandins Leukot Essent Fatty Acids 33(1):1–6. https://doi.org/10.1016/0952-3278(88)90115-9
Boskabady MH, Shaikhi J (2000) Inhibitory effect of Carum copticum on histamine (H1) receptors of isolated guinea-pig tracheal chains. J Ethnopharmacol 69(3):217–227. https://doi.org/10.1016/s0378-8741(99)00116-6
Agrewala JN, Pant MC (1986) Effect of feeding Carum copticum seeds on serum lipids, high density lipoproteins & serum cholesterol binding reserve in the albino rabbits. Indian J Med Res 83:93–95
Gilani AH, Jabeen Q, Ghayur MN, Janbaz KH, Akhtar MS (2005) Studies on the antihypertensive, antispasmodic, bronchodilator and hepatoprotective activities of the Carum copticum seed extract. J Ethnopharmacol 98(1–2):127–135. https://doi.org/10.1016/j.jep.2005.01.017
Zahin M, Ahmad I, Aqil F (2010) Antioxidant and antimutagenic activity of Carum copticum fruit extracts. Toxicol In Vitro 24(4):1243–1249. https://doi.org/10.1016/j.tiv.2010.02.004
Ahsan SK, Shah AH, Tanira MOM, Ahmad MS, Tariq M, Ageel AM (1990) Studies on some herbal drugs used against kidney stones in Saudi folk medicine. Fitoterapia 61(5):435–438
Kaur T, Bijarnia RK, Singla SK, Tandon C (2009) In vivo efficacy of Trachyspermum ammi anticalcifying protein in urolithiatic rat model. J Ethnopharmacol 126(3):459–462. https://doi.org/10.1016/j.jep.2009.09.015
Williamson EM, Okpako DT, Evans FJ (1996) Selection, preparation, and pharmacological evaluation of plant material. John Wiley & Sons
Khan A, Bashir S, Gilani AH (2012) An in vivo study on the diuretic activity of Holarrhena antidysenterica. Afr J Pharm Pharmacol 6(7):454–458
Khan A, Gilani AH (2010) Pharmacological basis for the medicinal use of Origanum vulgare Linn. In: Urolithiasis [abstract]. Paper presented at the 16th world congress of basic and clinical pharmacology, Copenhagen, Denmark
Khan A, Khan SR, Gilani AH (2012) Studies on the in vitro and in vivo antiurolithic activity of Holarrhena antidysenterica. Urol Res 40(6):671–681. https://doi.org/10.1007/s00240-012-0483-1
Pizzolato P (1971) Mercurous nitrate as a histochemical reagent for calcium phosphate in bone and pathological calcification and for calcium oxalate. Histochem J 3(6):463–469. https://doi.org/10.1007/BF01014785
Hess B, Meinhardt U, Zipperle L, Giovanoli R, Jaeger P (1995) Simultaneous measurements of calcium oxalate crystal nucleation and aggregation: impact of various modifiers. Urol Res 23(4):231–238. https://doi.org/10.1007/BF00393304
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193(1):265–275
Ebisuno S, Komura T, Yamagiwa K, Ohkawa T (1997) Urease-induced crystallizations of calcium phosphate and magnesium ammonium phosphate in synthetic urine and human urine. Urol Res 25(4):263–267. https://doi.org/10.1007/BF00942096
Guerra A, Meschi T, Allegri F, Schianchi T, Adorni G, Novarini A, Borghi L (2004) Calcium oxalate crystallization in untreated urine, centrifuged and filtered urine and ultrafiltered urine. Clin Chem Lab Med 42(1):45–50. https://doi.org/10.1515/CCLM.2004.009
Huang DJ, Chen HJ, Hou WC, Lin CD, Lin YH (2004) Active recombinant thioredoxin h protein with antioxidant activities from sweet potato (Ipomoea batatas [L.] Lam Tainong 57) storage roots. J Agric Food Chem 52(15):4720–4724. https://doi.org/10.1021/jf0498618
Borchert VE, Czyborra P, Fetscher C, Goepel M, Michel MC (2004) Extracts from Rhois aromatica and Solidaginis virgaurea inhibit rat and human bladder contraction. Naunyn Schmiedebergs Arch Pharmacol 369(3):281–286. https://doi.org/10.1007/s00210-004-0869-x
Ajith TA, Usha S, Nivitha V (2007) Ascorbic acid and alpha-tocopherol protect anticancer drug cisplatin induced nephrotoxicity in mice: a comparative study. Clin Chim Acta 375(1–2):82–86. https://doi.org/10.1016/j.cca.2006.06.011
Farre AJ, Colombo M, Fort M, Gutierrez B (1991) Differential effects of various Ca2+ antagonists. Gen Pharmacol 22(1):177–181. https://doi.org/10.1016/0306-3623(91)90331-y
Van Rossum JM (1963) Cumulative dose-response curves. II. Technique for the making of dose-response curves in isolated organs and the evaluation of drug parameters. Arch Int Pharmacodyn Ther 143:299–330
Kalaiselvi P, Udayapriya KL, Selvam R (1999) Uric acid-binding proteins in calcium oxalate stone formers and their effect on calcium oxalate crystallization. BJU Int 83(9):919–923. https://doi.org/10.1046/j.1464-410x.1999.00084.x
Dasaeva LA, Shilov EM, Shatokhina SN (2003) Diuress for the treatment of early stages of urolithiasis. Klin Med (Mosk) 81(10):50–52
Goldfarb DS, Coe FL (2005) The medical management of stone disease. In: Davison AM, Cameron JS, Ritz E, Grünfeld J, Winearls CG, Ponticelli C, van Ypersele C (eds) Oxford textbook of clinical nephrology. Oxford University Press, New York, pp 1199–1279
Tsai CH, Chen YC, Chen LD, Pan TC, Ho CY, Lai MT, Tsai FJ, Chen WC (2008) A traditional Chinese herbal antilithic formula, Wulingsan, effectively prevents the renal deposition of calcium oxalate crystal in ethylene glycol-fed rats. Urol Res 36(1):17–24. https://doi.org/10.1007/s00240-007-0122-4
Vanachayangkul P, Chow N, Khan SR, Butterweck V (2011) Prevention of renal crystal deposition by an extract of Ammi visnaga L. and its constituents khellin and visnagin in hyperoxaluric rats. Urol Res 39(3):189–195. https://doi.org/10.1007/s00240-010-0333-y
Bashir S, Gilani AH (2011) Antiurolithic effect of berberine is mediated through multiple pathways. Eur J Pharmacol 651(1–3):168–175. https://doi.org/10.1016/j.ejphar.2010.10.076
Fan J, Glass MA, Chandhoke PS (1999) Impact of ammonium chloride administration on a rat ethylene glycol urolithiasis model. Scanning Microsc 13(2–3):299–306
Park HK, Jeong BC, Sung MK, Park MY, Choi EY, Kim BS, Kim HH, Kim JI (2008) Reduction of oxidative stress in cultured renal tubular cells and preventive effects on renal stone formation by the bioflavonoid quercetin. J Urol 179(4):1620–1626. https://doi.org/10.1016/j.juro.2007.11.039
Thamilselvan S, Khan SR, Menon M (2003) Oxalate and calcium oxalate mediated free radical toxicity in renal epithelial cells: effect of antioxidants. Urol Res 31(1):3–9. https://doi.org/10.1007/s00240-002-0286-x
Wiessner JH, Hasegawa AT, Hung LY, Mandel GS, Mandel NS (2001) Mechanisms of calcium oxalate crystal attachment to injured renal collecting duct cells. Kidney Int 59(2):637–644. https://doi.org/10.1046/j.1523-1755.2001.059002637.x
Tiselius HG (2003) Epidemiology and medical management of stone disease. BJU Int 91(8):758–767. https://doi.org/10.1046/j.1464-410x.2003.04208.x
Wesson JA, Worcester EM, Wiessner JH, Mandel NS, Kleinman JG (1998) Control of calcium oxalate crystal structure and cell adherence by urinary macromolecules. Kidney Int 53(4):952–957. https://doi.org/10.1111/j.1523-1755.1998.00839.x
Wesson JA, Ward MD (2006) Role of crystal surface adhesion in kidney stone disease. Curr Opin Nephrol Hypertens 15(4):386–393. https://doi.org/10.1097/01.mnh.0000232879.50716.6f
Guerra A, Meschi T, Allegri F, Prati B, Nouvenne A, Fiaccadori E, Borghi L (2006) Concentrated urine and diluted urine: the effects of citrate and magnesium on the crystallization of calcium oxalate induced in vitro by an oxalate load. Urol Res 34(6):359–364. https://doi.org/10.1007/s00240-006-0067-z
Wang AY (2009) Vascular and other tissue calcification in peritoneal dialysis patients. Perit Dial Int 29(Suppl 2):S9–S14
Khan SR (1997) Animal models of kidney stone formation: an analysis. World J Urol 15(4):236–243. https://doi.org/10.1007/BF01367661
Marangella M, Bagnis C, Bruno M, Vitale C, Petrarulo M, Ramello A (2004) Crystallization inhibitors in the pathophysiology and treatment of nephrolithiasis. Urol Int 72(Suppl 1):6–10. https://doi.org/10.1159/000076583
Carvalho M, Vieira MA (2004) Changes in calcium oxalate crystal morphology as a function of supersaturation. Int Braz J Urol 30(3):205–208. https://doi.org/10.1590/s1677-55382004000300005
Kato Y, Yamaguchi S, Yachiku S, Nakazono S, Hori J, Wada N, Hou K (2004) Changes in urinary parameters after oral administration of potassium-sodium citrate and magnesium oxide to prevent urolithiasis. Urology 63(1):7–11. https://doi.org/10.1016/j.urology.2003.09.057
Aihara K, Byer KJ, Khan SR (2003) Calcium phosphate-induced renal epithelial injury and stone formation: involvement of reactive oxygen species. Kidney Int 64(4):1283–1291. https://doi.org/10.1046/j.1523-1755.2003.00226.x
Escobar C, Byer KJ, Khaskheli H, Khan SR (2008) Apatite induced renal epithelial injury: insight into the pathogenesis of kidney stones. J Urol 180(1):379–387. https://doi.org/10.1016/j.juro.2008.02.041
Byer K, Khan SR (2005) Citrate provides protection against oxalate and calcium oxalate crystal induced oxidative damage to renal epithelium. J Urol 173(2):640–646. https://doi.org/10.1097/01.ju.0000143190.49888.c7
Santhosh Kumar M, Selvam R (2003) Supplementation of vitamin E and selenium prevents hyperoxaluria in experimental urolithic rats. J Nutr Biochem 14(6):306–313. https://doi.org/10.1016/s0955-2863(03)00033-0
Babich H (1982) Butylated hydroxytoluene (BHT): a review. Environ Res 29(1):1–29. https://doi.org/10.1016/0013-9351(82)90002-0
Ohgaki K, Horiuchi K, Hikima N, Kondo Y (2010) Facilitation of expulsion of ureteral stones by addition of alpha1-blockers to conservative therapy. Scand J Urol Nephrol 44(6):420–424. https://doi.org/10.3109/00365599.2010.497769
Seitz C, Liatsikos E, Porpiglia F, Tiselius HG, Zwergel U (2009) Medical therapy to facilitate the passage of stones: what is the evidence? Eur Urol 56(3):455–471. https://doi.org/10.1016/j.eururo.2009.06.012
Bolton TB (1979) Mechanisms of action of transmitters and other substances on smooth muscle. Physiol Rev 59(3):606–718. https://doi.org/10.1152/physrev.1979.59.3.606
Godfraind T, Miller R, Wibo M (1986) Calcium antagonism and calcium entry blockade. Pharmacol Rev 38(4):321–416
Brown JH, Taylor P (2006) Muscarinic receptor agonists and antagonists. Goodman and Gilman’s manual of pharmacology and therapeutics, 11th edn. McGraw-Hill Professional, New York, pp 183–200
Fleckenstein A (1977) Specific pharmacology of calcium in myocardium, cardiac pacemakers, and vascular smooth muscle. Annu Rev Pharmacol Toxicol 17(1):149–166. https://doi.org/10.1146/annurev.pa.17.040177.001053
Acknowledgements
This study was supported by the Higher Education Commission (HEC) of Pakistan as (i) Indigenous M.Phill/PhD and (ii) International Research Support Initiative Program (IRSIP) scholarships awarded to Aslam Khan for carrying out research at Department of Biological and Biomedical Sciences, Aga Khan University Medical College, Karachi, Pakistan and Center for the Study of Lithiasis, Colleges of Medicine, University of Florida, Fl, USA, respectively.
Author information
Authors and Affiliations
Contributions
Dr. Anwarul-Hassan Gilani, supervised the research work and critically revise and correct the manuscript. Dr. Aslam Khan, did the experimental work and wrote the first draft of the mannuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khan, A., Gilani, A.H. An insight investigation to the antiurolithic activity of Trachyspermum ammi using the in vitro and in vivo experiments. Urolithiasis 51, 43 (2023). https://doi.org/10.1007/s00240-023-01415-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00240-023-01415-9